Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
InVivoKines™ - Proteins with Enhanced Activity & Stability for InVivo Research
Cytokines are small proteins that facilitate communication among immune cells and orchestrate the response to infections and tumors as well as overall immune homeostasis, making them attractive for preclinical and clinical research for a variety of immune-related disorders. They have pivotal roles in immunity and engineered cytokine-based therapies represent a new evolution of immunotherapeutics. However, the widespread use of cytokines has been limited by their short blood half-lives, pleiotropism and unfavorable biodistribution. Increased knowledge of cytokine biology and innovative cytokine engineering and technologies allow to cope with such limitations but often are not accessible for basic research purposes.
InVivoKines™ are a new generation of recombinant fusion proteins for immunotherapeutic, preclinical and translational in vivo research, developed in-house by AdipoGen Life Sciences. InVivoKines™ are Fc-based fusion proteins using the Knobs-into-Holes (KIH) technology.
InVivoKines™ Quality Features
• Native Conformation − Production in HEK 293 or CHO Cells
• Production under Animal-Free Conditions
• High Bioactivity tested by ELISA/Cell-based Assays
• Verified Purity & Homogeneity by SEC
• Low Endotoxin Levels
• Batch-to-Batch Consistency
More Details About
• KIH-Technology
• Silenced Fc-KIH Domain (LALA-PG Mutations)
• Potent Monomeric IL-2 Proteins
• NEW Highly Active Heterodimeric IL-27-KIH Protein
• NEW Unique Active Homodimeric GDF15-KIH Protein
• InVivoKines™ − Product Overview
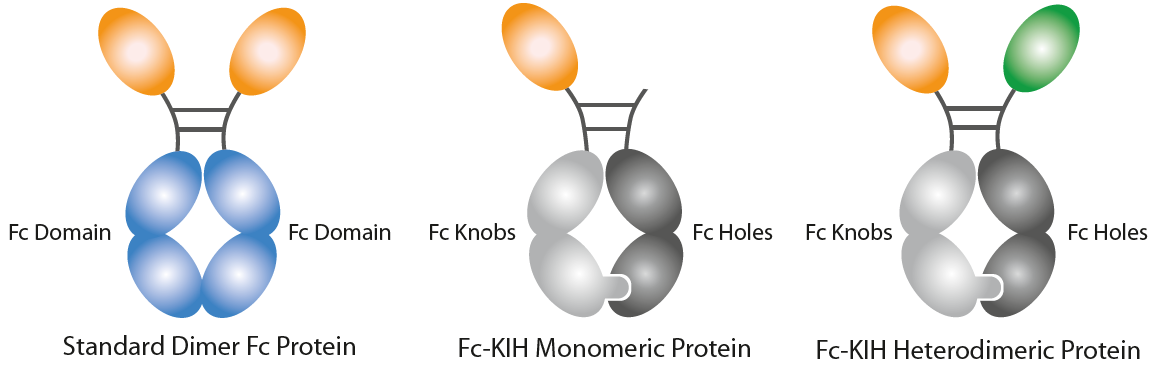
Naturally Occuring Cytokines Fused to Fc (KIH-Technology)InVivoKines™ are Fc-based fusion proteins using the Knobs-into-Holes (KIH) technology. • The Fc-KIH technology allows Fc heterodimerization to create a structure with two different arms, to design naturally occurring active monomeric, heterodimeric or homodimeric proteins. AdipoGen Life Sciences leveraged the KIH-technology to develop monomeric, heterodimeric or homodimeric Fc fusion proteins. *Antibody-dependent cellular cytotoxicity (ADCC), Antibody-dependent cellular phagocytosis (ADCP), Complement-dependent cytotoxicity (CDC) |
|
|
|
Background on the Knobs-into-Holes Technology: The Knobs-into-Holes (KIH) concept/format was a pioneering format proposed by Ridgway et al. in 1996, permitting heavy-chain heterodimerization, creating an ‘IgG with two different arms’, and traditionally it is used to engineer bispecific antibodies with an important role in drug development. It allows the generation of complementary interacting interfaces by manipulating key amino acid residues that participate in the Fc dimeric interaction. Amino acids with a small side chain are replaced by ones with larger side chains, thereby creating a knob or protrusion in one chain and vice versa to create a hole or socket in the partner chain. Traditionally, a T366Y mutation in one CH3 domain has been used to create a knob while an Y407T mutation in the other CH3 domain gives rise to a hole. Such mutations establish intermolecular interactions and promote the heterodimer formation due to knob/hole pairing. Production of KIH bispecific biologicals to high purity is challenging due to the inherent complexity of the molecule. The efficient co-expression of KIH relies on approximately equal expression of both knob and hole. Therefore, the selection of a stable expression clone is a prerequisite for KIH production using mammalian cells and is a laborious and time-consuming process. |
Silenced Fc-KIH Domain (LALA-PG Mutations)AdipoGen Life Sciences engineers its silenced Fc-KIH domains using the hIgG1-P329G LALA mutations, with completely abolished FcγR and C1q interactions, containing a limited number of mutations and with unaffected FcRn interactions and Fc stability. The LALA-PG mutations show no detectable binding to Fcγ receptors or to C1q, are inactive in functional cell-based assays and do not elicit inflammatory cytokine responses. |
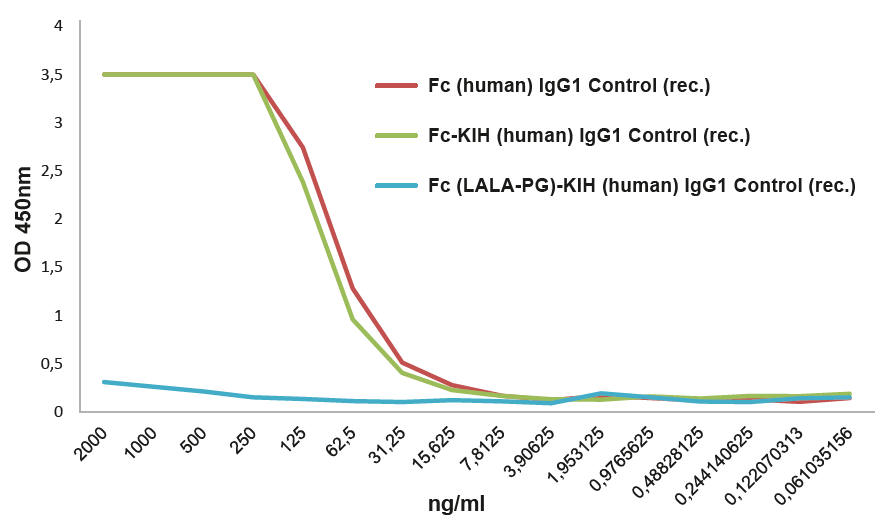 |
FIGURE: Fc (LALA-PG)-KIH (human) IgG1 Control (rec.) (Prod. No. AG-35B-0018) does not bind to the human Fcγ Receptor I. |
|
LITERATURE: (1) Knobs-into-Holes (KIH) Technology: J.B. Ridgway, et al.; Protein Eng. 9, 617 (1996) • (2) Efficient Generation of Bispecific Murine Antibodies for Pre-Clinical Investigations in Syngeneic Rodent Models: A.F. Labrijn, et al.; Sci. Rep. 7, 2476 (2017) • (3) Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions: T. Schlothauer, et al.; Prot. Eng. Design Sel. 29, 457 (2016) |
|
| Product Name | PID | Protein Construct | Fc-KIH Silenced |
| Fc-KIH (human) IgG1 Control (rec.) | AG-35B-0015 | Control | No |
| Fc (LALA-PG)-KIH (human) IgG1 Control (rec.) | AG-35B-0018 | Control | LALA-PG |
|
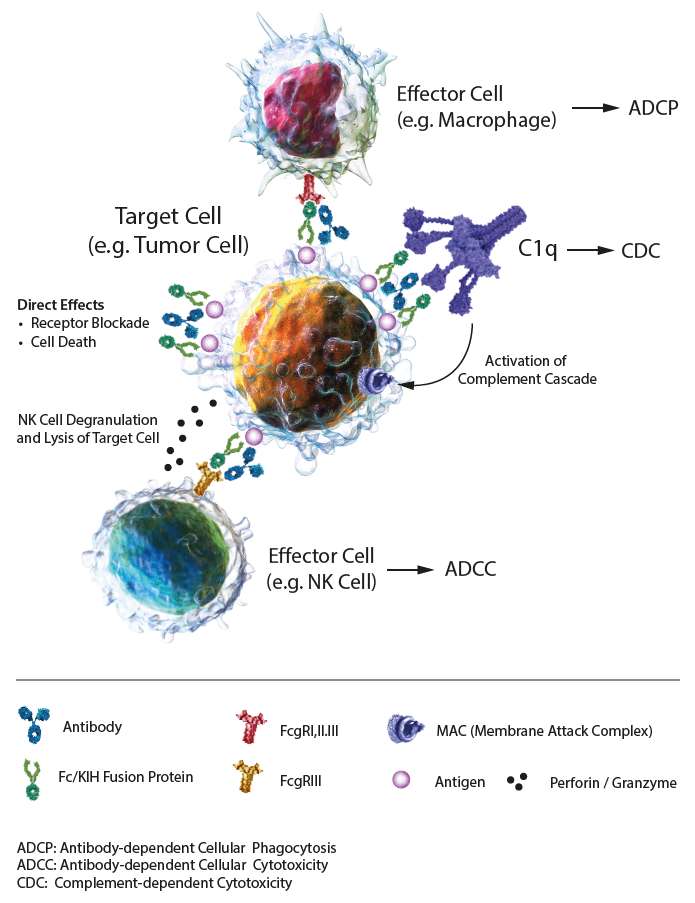
IgG-based Effector Functions IgG-based antibodies and Fc fusion proteins are biologicals exploited in preclinical and therapeutic applications. Their communication with the immune system is controlled and mediated by Fc gamma receptors (FcγRs), membrane-bound proteins. These biologicals are composed of an antigen-binding site, thereby determining the specificity and affinity and the Fc segment that can bind to Fc receptors (FcγRI, FcγRII, FcγRIII) expressed on the surface of immune cells, complement (C1q) and FcRn (neonatal FcR) in the blood, thereby activating the immune effect. Effector functions mediated by the Fc domain of the biologicals can strongly influence the functional outcome, through antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC), all mediated through the interaction of the Fc domain with Fc receptors on different cell types. ADCC: FcRs on effector cells bind to the Fc region of biologicals on a target cell, resulting in secretion of lytic enzymes and the expression of cell death-inducing molecules, inducing target cell lysis. ADCP: FcRs on phagocytic cells bind to the Fc region of biologicals on a target cell or immune complex, promoting clearance via phagocytosis. CDC: C1q binds to the Fc domain of biologicals on a target cell, triggering the complement cascade and assembly of the membrane attack complex (MAC), resulting in cell lysis. Half-life: The combination of IgG/Fc domains (IgGs) and neonatal FcR (FcRn) can protect antibodies and Fc fusion proteins from being degraded, thereby prolonging the half-life in serum. Under acidic conditions (pH 6.0-6.5), FcRn binds IgGs, and dissociation occurs under neutral and weakly alkaline conditions (pH 7.0-7.5). Specifically, endothelial cells form endocytosis vesicles by ingesting IgGs to form an acidified endosome and IgGs bind to FcRn to form an IgGs-FcRn complex. The IgGs-FcRn complex is transported to the cell surface through the recirculated endosome. Under physiological conditions such as pH 7.4, the IgGs-FcRn complex dissociates, and the IgG is released again into the blood circulation. Through this receptor-mediated recycling mechanism, FcRn effectively protects IgGs from lysosomal degradation, thus prolonging the half-life of IgGs. The Knobs-into-Holes (KIH)-Fc domains show similar specifications as standard Fc fusion domains or antibody IgGs. LITERATURE: The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies: P. Gogesch, et al.; Int. J. Mol. Sci. 22, 8947 (2021) |
FIGURE: Mechanisms of action of IgG1 Abs or Fc fusion proteins, composed of direct effects, and indirect effects, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC). |
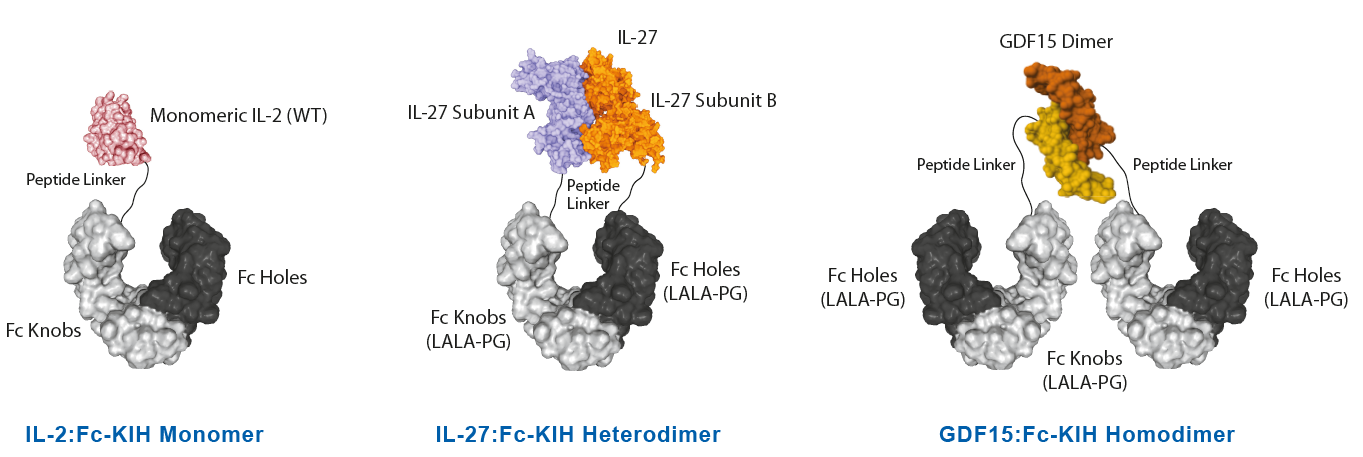
Potent Monomeric IL-2 ProteinsIL-2 is a key lymphocyte growth factor that is required for the maintenance and expansion of effector T cells (CD4 and CD8), regulatory T cells (Tregs) and natural killer (NK) cells. IL-2 signals through intermediate-affinity receptors that comprise IL-2Rβ and IL-2Rγ and high-affinity receptors that also contain IL-2Rα (CD25). IL-2 is critical for the activation and proliferation of T cells, commonly used in TIL cell therapy to promote T cell expansion. The IL-2 Superkine has been shown to induce T cell (CD4/CD8) proliferation (not Treg), leading to superior expansion of cytotoxic CD8 T cells and NK cells, and consequently to improved antitumor response in vivo compared to human IL-2 WT. IL-2 Superkine (monomeric):Fc-KIH is much more potent compared to IL-2 Superkine:Fc (dimeric) and equivalent or better than the Gold Standard IL-2 Aldesleukin. |
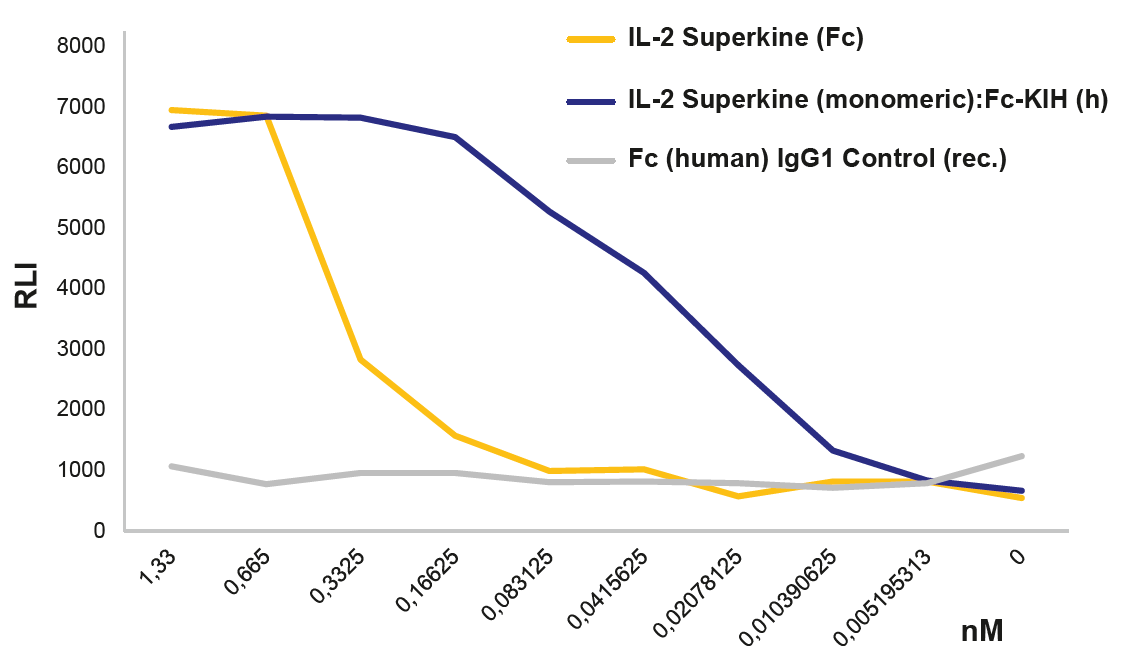 |
FIGURE: IL-2 Superkine (monomeric):Fc-KIH (human) (rec.) (Prod. No. AG-40B-0222) activates the IL-2 receptor better than the dimeric IL-2 Superkine (Prod. No. AG-40B-0111) using the IL-2 Bioassay (Promega JA2201). |
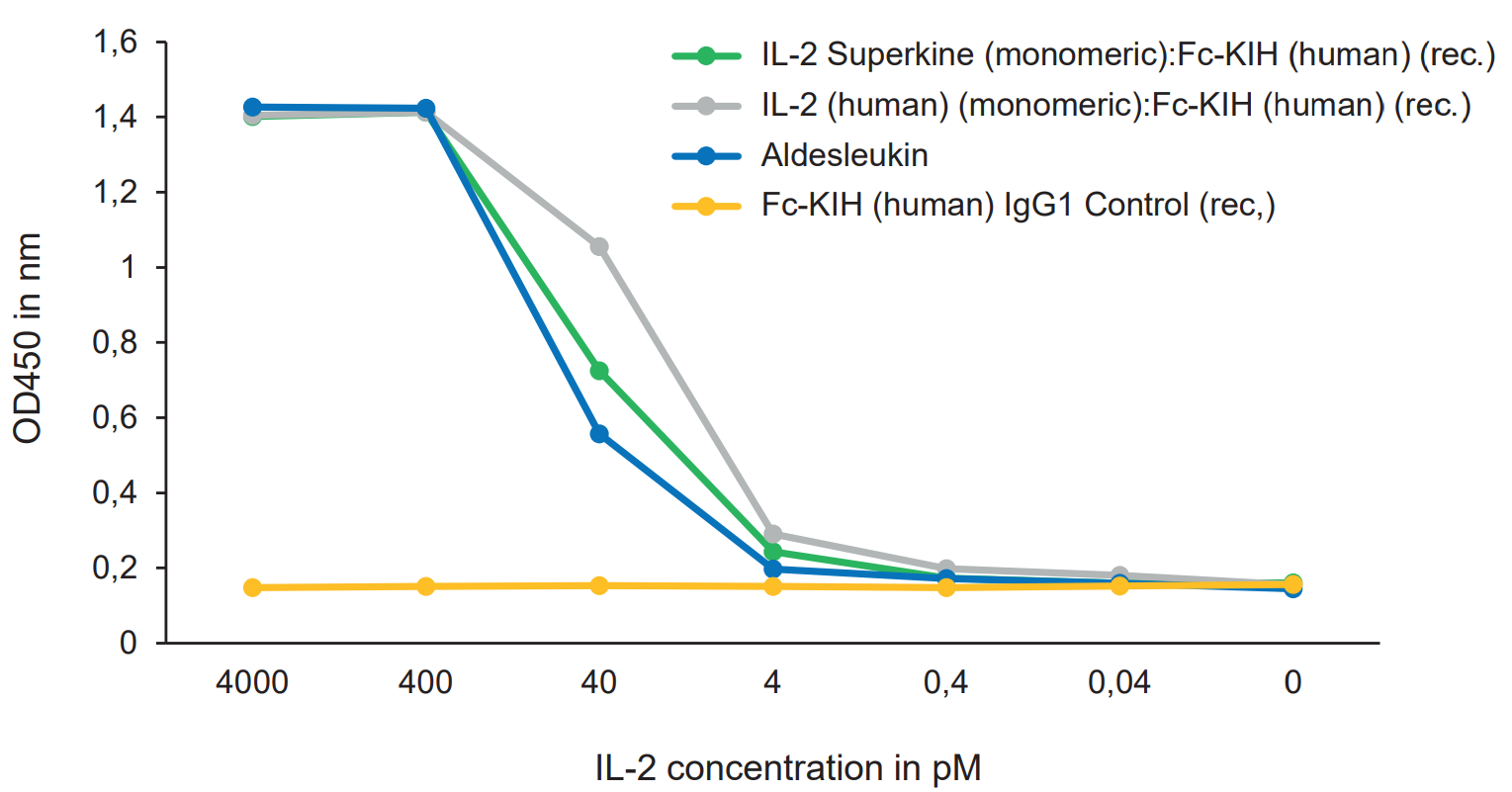 |
FIGURE: IL-2 Superkine (monomeric):Fc-KIH (human) (rec.) (Prod. No. AG-40B-0222) and IL-2 (human) (monomeric):Fc-KIH (human) (rec.) (Prod. No. AG-40B-0224) are equivalent or better than the Gold Standard IL-2 Aldesleukin to increase proliferation of CTLL-2 cytotoxic T cells. |
| Product Name | PID | Protein Construct | Fc-KIH Silenced |
| IL-2 Superkine (monomeric):Fc-KIH (human) (rec.) | AG-40B-0222 | Monomer | No |
| IL-2 Superkine H9T (monomeric):Fc-KIH (human) (rec.) | AG-40B-0223 | Monomer | No |
| IL-2 (human) (monomeric):Fc-KIH (human) (rec.) | AG-40B-0224 | Monomer | No |
| IL-2 (mouse) (monomeric):Fc-KIH (human) (rec.) | AG-40B-0225 | Monomer | No |
NEW Highly Active Heterodimeric IL-27-KIH ProteinIL-27 is composed of two subunits, IL-27p28 and EBI3. It promotes NK and T cell proliferation as well as the production of IFN-γ. IL-27 has potent antiviral activities against numerous viruses and antitumor activity via production of anti-angiogenic chemokines. IL-27 (mouse):Fc-KIH (human) (rec.) is a heterodimeric Fc-KIH construct that binds to mouse & human IL-27R complex and activates Stat3 phosphorylation in mouse and human cells. Its activity is stronger compared to the classical dimeric IL-27 (mouse):Fc from competitors. |
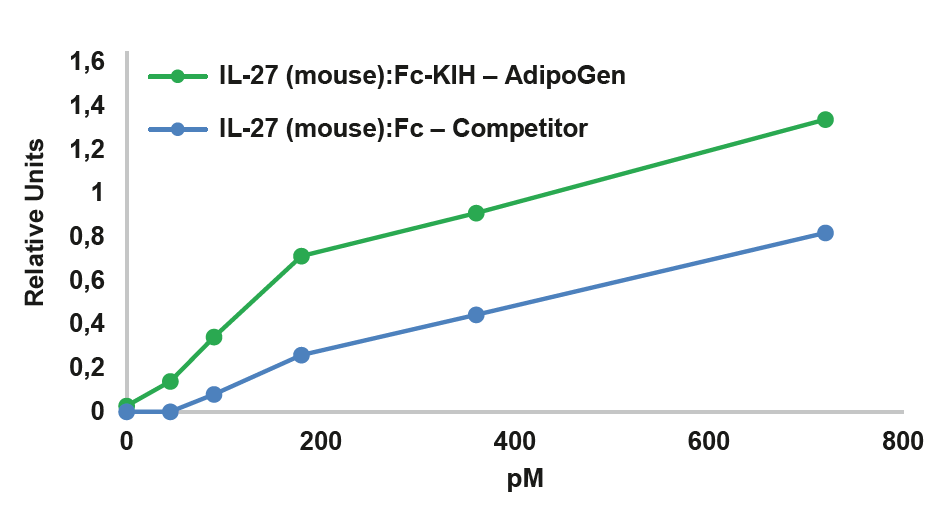 |
FIGURE: IL-27 (mouse):Fc-KIH (human) (rec.) (Prod. No. AG-40B-0236) (Heterodimer) is more active than an IL-27 (mouse):Fc from a competitor (Dimer of Heterodimer) in Stat3 phosphorylation in HepG2 cells (shown as quantification figure of the western blot). |
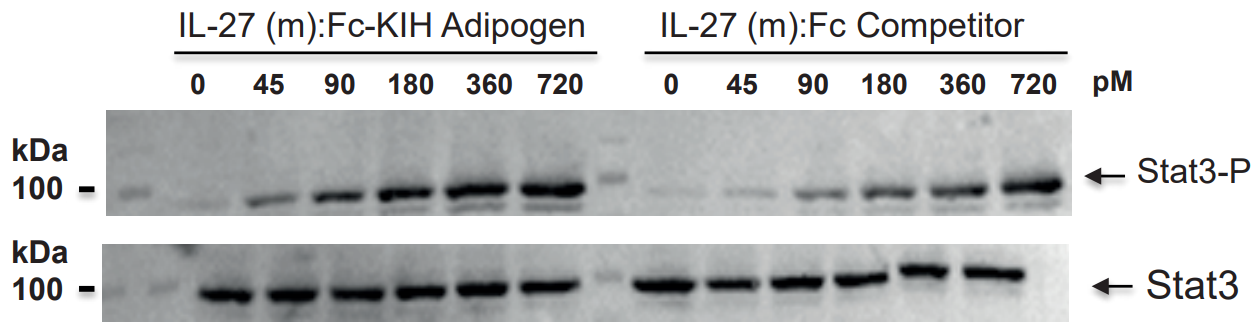 |
FIGURE: IL-27 (mouse):Fc-KIH (human) (rec.) (Prod. No. AG-40B-0236) (Heterodimer) is more active than an IL-27 (mouse):Fc from a competitor (Dimer of Heterodimer) in Stat3 phosphorylation in HepG2 cells. |
| Product Name | PID | Protein Construct | Fc-KIH Silenced |
| IL-27 (mouse):Fc-KIH (human) (rec.) | AG-40B-0236 | Heterodimer | No |
| IL-27 (mouse):Fc (LALA-PG)-KIH (human) (rec.) | AG-40B-0249 | Heterodimer | LALA-PG |
NEW Unique Active Homodimeric GDF15-KIH ProteinThe effects of GDF15 are pleiotropic and include appetite regulation, actions on metabolism, pregnancy, cell survival, immune response and inflammation. AdipoGen Life Sciences' homodimeric protein Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) is a unique protein with long-acting high activity for in vivo studies. It forms a biologically active homodimer, unlike other classical GDF15-Fc proteins that form inactive large aggregates. |
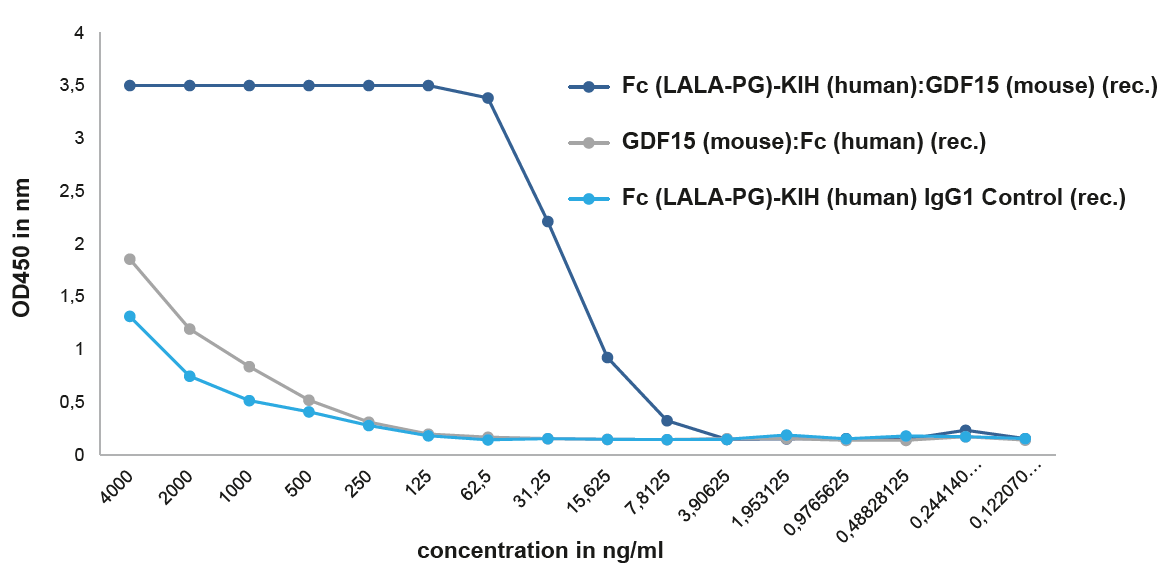 |
FIGURE: A binding assay shows that Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) (Prod. No. AG-40B-0245) binds with high affinity to its receptor GFRAL (mouse). The Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.), which migrates as a dimer by SEC, binds to its receptor mouse GFRAL with an EC50 ~15 ng/ml, while the GDF15 (mouse):Fc (human) (rec.), that migrates as an aggregate by SEC (>1000 kDa), binds similar to the control protein with low affinity. . |
| Product Name | PID | Protein Construct | Fc-KIH Silenced |
| Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) | AG-40B-0245 | Homodimer | LALA-PG |
InVivoKines™ - Selected Structures |