Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
GDF15, an Exercise-induced Factor regulating Appetite & Energy Expenditure
Growth and differentiation factor 15 (GDF15, Macrophage Inhibitory Cytokine 1; MIC-1) is a member of transforming growth factor-β (TGF-β) superfamily and was initially identified in activated macrophages. Similar to other TGF-β family proteins, GDF15 is synthesized as a large precursor protein that is cleaved to release the mature protein that shares 66% and 97% amino acid sequence identity with the human and rat proteins, respectively. Biologically active GDF15 is a disulfide-linked homodimer of the mature protein.
GDF15 acts through a recently identified receptor expressed in the brain called glial-derived neurotrophic factor (GDNF) receptor α-like (GFRAL) which signals through the "Rearranged during Transfection" (RET) tyrosine kinase receptor. Upon binding to GFRAL-RET, GDF15 causes activation of Erk1/2 (extracellular signal-regulated kinase), Akt (Ak strain transforming) and PLC-γ (phospholipase C-γ). GDF15 is highly expressed in the placenta and brain, and it is expressed at lower levels in the kidney, pancreas, prostate and colon. It should be noted that physiological effects of GDF-15 comprise many actions on cell types without detectable GFRAL expression, suggesting that GFRAL-independent effects of GDF-15 may also exist.
GDF15 serum levels rise in response to cell stress, to act locally in an autocrine or paracrine manner. GDF15 is secreted at elevated levels from a variety of cell types including tumor and inflammatory cells, in response to various stresses including chronic inflammation, obesity, cardiovascular diseases, cancers and chemotherapy treatment. GDF15 concentrations also increase with age. Circulating GDF15 increases during exercise and recovery from exercise in humans.
Biological Relevance of GDF15
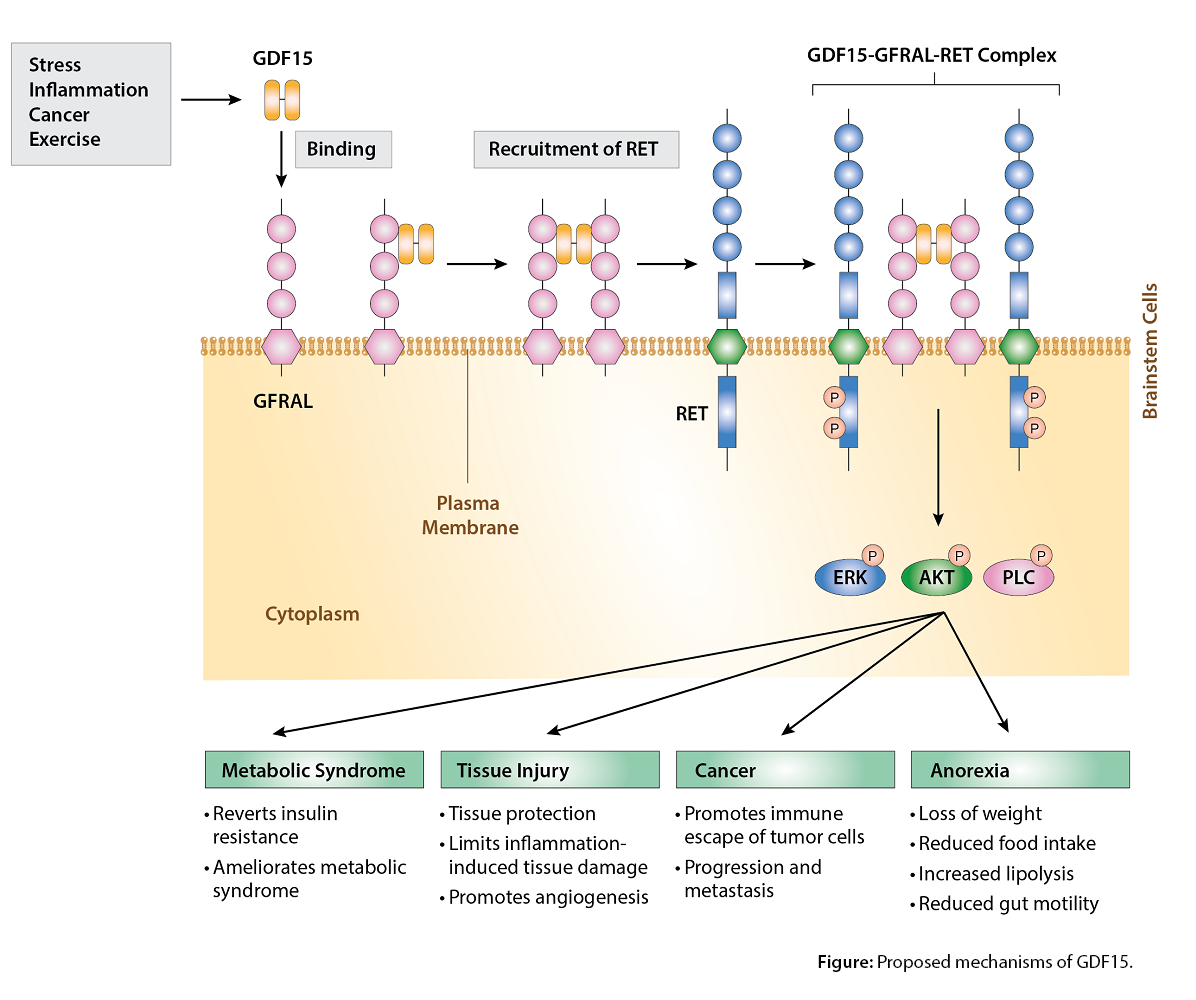
The effects of GDF15 are pleiotropic and include appetite regulation, actions on metabolism, pregnancy, cell survival, immune response and inflammation. GDF-15 also plays different roles in the pathophysiology of different tissues, such as cardiovascular and liver disease, autoimmunity, cancer progression/metastasis and metabolic syndrome/diabetes (see Figure).
High levels of GDF15 cause anorectic effects and cachexia, largely if not exclusively through the suppression of food intake via modulation of neuropeptide Y and pro-opiomelanocortin levels. GDF15 promotes also neuronal survival. GDF15/GFRAL signaling is implicated in diet-based obesity and insulin resistance. GDF15 is cardioprotective via inhibition of platelet activation, limiting atherosclerosis, promoting recovery following myocardial infarction and regulating angiogenesis.
These actions are associated with reduction of inflammatory process and insulin resistance. GDF15 emerges as a relevant contributor in the energy-homeostasis field, and it appears as a biomarker of various cardiovascular and metabolic diseases. By modifying the amount of GDF15-GFRAL-RET signaling, a new class of GDF15/GFRAL/RET-based drugs is emerging.
GDF15, a circulating protein activated by stress and inflammation, links as a predictive biomarker with multiple pathological processes including obesity, diabetes, metabolic syndrome, heart failure, atherosclerosis, neurodegeneration, inflammatory diseases and cancers. It has also been implicated in the nausea and vomiting of pregnancy (NVP) including its most severe form, Hyperemesis Gravidarum (HG), where it has been suggested that the hormone-caused aversion to some smells and tastes might encourage an expectant parent to avoid foods potentially dangerous to the fetus.
Experimental and clinical studies support the potential use of GDF15 as a novel therapeutic target in relation to: (i) preventing and treating obesity by modulating metabolic activity; (ii) promoting adaptive angiogenesis; (iii) promoting regenerative processes; and (iv) desensitizing during pregnancy.
Literature References:
- Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets: L. Rochette, et al.; Trends Endocrinol. Metabol. 31, 939 (2020)
- Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint: J. Wischhusen, et al.; Front. Immunol. 11, 951 (2020)
- GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease: D. Wang, et al.; Nat. Rev. Endocrinol. 17, 592 (2021)
- GDF15 in Appetite and Exercise: Essential Player or Coincidental Bystander? A.B. Klein, et al.; Endocrinol. 163, bqab242 (2022)
- Role of growth differentiation factor 15 in cancer cachexia: T. Ling, et al.; Oncol. Lett. 26, 426 (2023)
- GDF15 linked to maternal risk of nausea and vomiting during pregnancy: M. Fejzo, et al.; Nature (Epub ahead of print) (2023)
Unique Biologically Active & Long-Acting GDF15 Homodimers
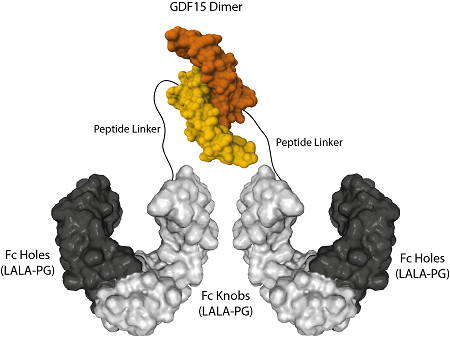
AdipoGen Life Sciences' homodimeric protein Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) is a unique protein with long-acting high activity for in vivo studies. It forms a biologically active homodimer, unlike other classical GDF15-Fc proteins that form large aggregates.
The protein Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) is produced by using two different vectors, one encoding for the Fc Knobs (LALA-PG) (human):GDF15 (mouse) sequence (synthesizing a protein of 45kDa) and one encoding for the Fc Holes (LALA-PG) sequence (synthesizing a protein of 30kDa). Both vectors transfected into HEK293 cells produce both Fc molecules (Knobs-into-Holes technology; J.B. Ridgway, et al.; Protein Eng. 9, 617 (1996)) required for dimerization and for secretion of the final protein Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.). This Fc-KIH format allows our mouse GDF15 protein to form a dimer that is the most active structure to bind and activate the GFRAL and RET receptor complex. The Fc contains the mutations LALA-PG that abolish the interaction between the Fc and FcγRs and therefore Fc undesirable effects.
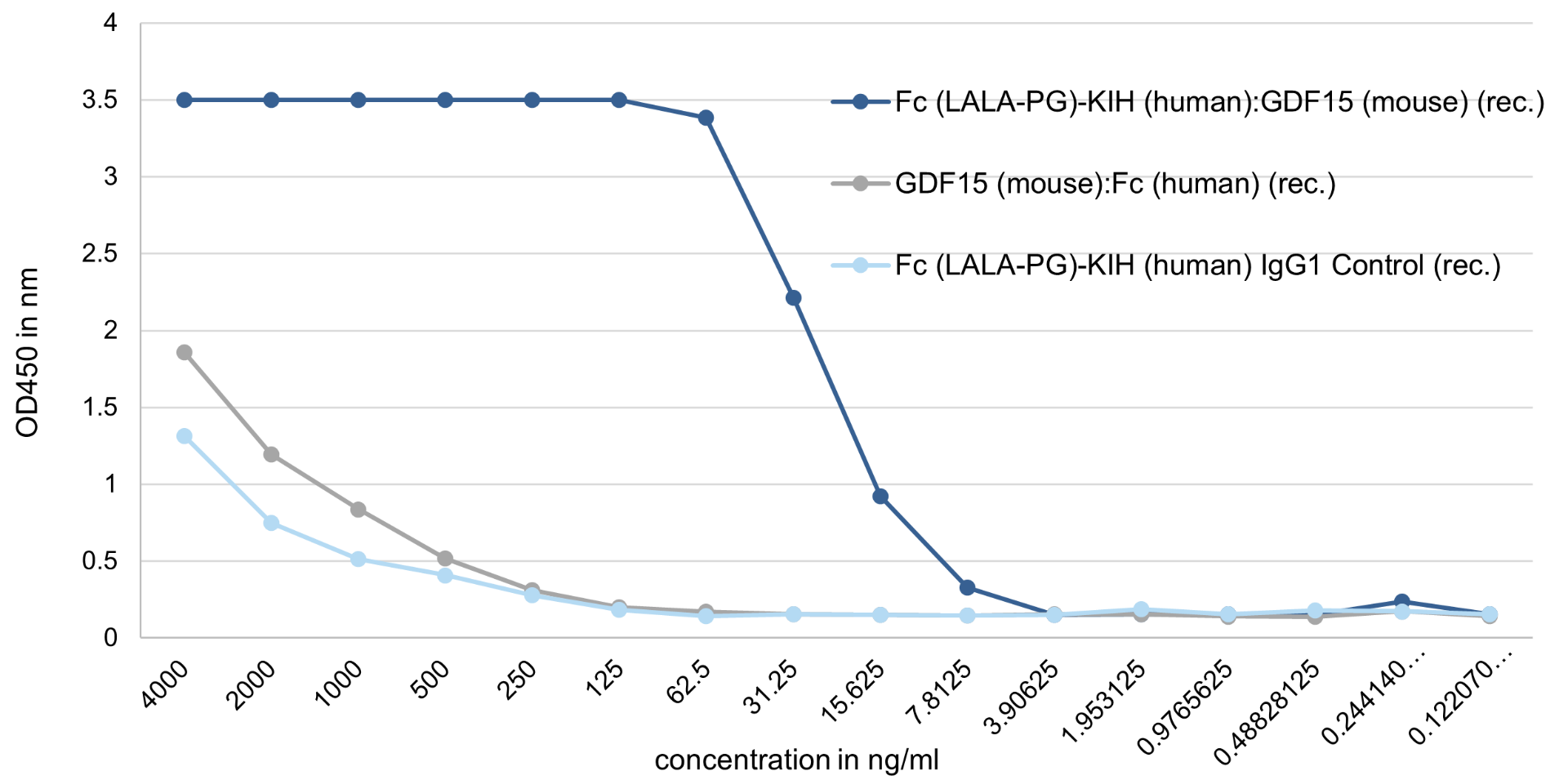
Binding Assay: Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) (AG-40B-0245) binds with high affinity to its receptor GFRAL (mouse).
Method: GFRAL (mouse) is coated on an ELISA plate at 500 ng/ml overnight at room temperature. Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.), a classical GDF15 (mouse):Fc (human) (rec.) (from competitor), or the control protein Fc (LALA-PG)-KIH (human) IgG1 Control (rec.) (AG-35B-0018) is added (starting at 4000 ng/ml with a twofold serial dilution) during one hour and then detected using a human Fc (HRP) antibody. The Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.), which migrates as a dimer by SEC, binds to its receptor mouse GFRAL with an EC50 ~15 ng/ml, while the GDF15 (mouse):Fc (human) (rec.), that migrates as an aggregate by SEC (>1000 kDa), binds similar to the control protein with low affinity.
Biologically Active GDF15 and GFRAL Proteins
| Product Name | PID | Source | Endotoxin | Species |
|
NEW Fc (LALA-PG)-KIH (human):GDF15 (mouse) (rec.) |
AG-40B-0245 | HEK 293 cells | <0.01EU/µg | Mouse |
| Fc (LALA-PG)-KIH (human) IgG1 Control (rec.) CONTROL PROTEIN | AG-35B-0018 | HEK 293 cells | <0.01EU/µg | Human, Mouse |
| GFRAL (human):Fc (human) (rec.) | CHI-HF-210GFRAL | HEK 293 cells | <1EU/mg | Human |
| GFRAL (mouse):Fc (mouse) (rec.) | CHI-MF-110GFRAL | HEK 293 cells | <0.06EU/µg | Mouse |