Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Innaxon
IAXO-102 (CD14/TLR4 Antagonist) (synthetic)

| Product Details | |
|---|---|
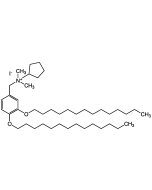
| Synonyms | Methyl 6-deoxy-6-amino-2,3-di-O-tetradecyl-α-D-glucopyranoside |
| Product Type | Chemical |
| Properties | |
| Formula |
C35H71NO5 |
| MW | 585.94g/mol |
| CAS | 1115270-63-7 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (TLC) |
| Appearance | White solid. |
| Solubility | Soluble in Methanol, DMSO and Ethanol 1:1 (vol:vol): >10mM. |
| Biological Activity |
Described to interfere with human, rat and mouse TLR4/CD14 signaling, other species not tested. Optimal working concentration depends upon the type, purity and concentration and of TLR4 ligand, carrier protein such as LPS-binding protein (LBP), soluble and membrane-bound CD14, the presence of TLR4 co-receptors (e.g. CD36) as well as on type and time of read-out (e.g. cytokine measurement in cell culture supernatant) or the biological outcome of in vivo experiments and therefore needs to be determined for each application. Recommended starting concentration: in vitro: 5μM, in vivo (rodent): 3mg/kg. |
| Identity | Determined by NMR and MS. |
| Declaration | Manufactured by Innaxon. |
| Other Product Data |
Reconstitution: For a 2mM stock solution, dissolve total vial content in 853μl (1mg size) or 4,266μl (5mg size) in (1:1) DMSO/Ethanol (vol:vol). |
| InChi Key | InChI=1/C35H71NO5/c1-4-6-8-10-12-14-16-18-20-22-24-26-28-39-33-32(37)31(30-36)41-35(38-3)34(33)40-29-27-25-23-21-19-17-15-13-11-9-7-5-2/h31-35,37H,4-30,36H2,1-3H3 |
| Smiles | O[C@H]1[C@H](OCCCCCCCCCCCCCC)[C@@H](OCCCCCCCCCCCCCC)[C@@H](OC)O[C@@H]1CN |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
- CD14/TLR4 antagonist. Inhibitor of sterile inflammation.
- Synthetic TLR4/CD14 ligand with TLR4 modulating activities in vitro, and conferring protection against TLR4/CD14-mediated tissue damage and inflammation in vivo. Useful to explore CD14- dependent and TLR4-independent pathways and TLR4 activation by endogenous ligands (e.g. hyaluronic acid oligosaccharides, oxLDL, HMGB1) in sterile inflammation. Shown to inhibit neuropathic pain, secondary necrosis of acute drug-induced liver failure and vascular inflammation, and abdominal aortic aneurysm by blocking non-hematopoietic TLR4 signaling. Useful tool, where inhibition of sterile (auto-) inflammation is desired, without compromising TLR4’s key role in the defense of pathogens.
- Inhibition of lipid a stimulated activation of human dendritic cells and macrophages by amino and hydroxylamino monosaccharides: F. Peri, et al.; Angew. Chem.46, 3308 (2007)
- TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice: I. Bettoni, et al.; Glia 56, 1312 (2008)
- Glycolipids and benzylammonium lipids as novel antisepsis agents: synthesis and biological characterization: M. Piazza, et al.; J. Med. Chem. 52, 1209 (2009)
- Evidence of a specific interaction between new synthetic antisepsis agents and CD14: M. Piazza, et al.; Biochemistry 48, 12337 (2009)
- Exploring the LPS/TLR4 signal pathway with small molecules: F. Peri, et al.; Biochem. Soc. Trans. 38, 1390 (2010) (Review)
- Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists: F. Peri & M. Piazza; Biotechnol. Adv. 30, 251 (2012) (Review)
- Synthetic molecules and functionalized nanoparticles targeting the LPS-TLR4 signaling: A new generation of immunotherapeutics: F. Peri, et al.; Pure Appl. Chem. 84, 97 (2012) (Review)
- Toll like receptor 4 antagonist prevents acetaminophen induced acute liver failure in mice: a novel therapeutic strategy: N. Shah, et al.; Gut 61, A28 (2012)
- Multivalent glycoconjugates as anti-pathogenic agents. A. Bernardi, et al.; Chem. Soc. Rev. 42, 4709 (2013) (Review)
- Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: an update: F. Peri & V. Calabrese; Med. Chem. 57, 3612 (2014) (Review)
- A novel small mimetic molecule TLR4 antagonist (IAXO-102) modulates TLR4 proinflammatory signalling and inhibits aortic aneurysms development: C. Huggins, et al.; Atherosclerosis 241, e53 (2015)
- Effects of Toll-Like Receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice: F. Kawakita, et al.; Mol. Neurobiol. 54, 6624 (2017)
- Selective Toll-Like Receptor 4 antagonists prevent acute blood-brain barrier disruption after subarachnoid hemorrhage in mice: T. Okada, et al.; Mol. Neurobiol. 56, 976 (2019)
- Cancer-derived VEGF-C increases chemokine production in lymphatic endothelial cells to promote CXCR2-dependent cancer invasion and MDSC recruitment: J.Y. Chen, et al.; Cancers 11, 1120 (2019)
- Increasing the chemical variety of small-molecule-based TLR4 modulators: An Overview: A. Romerio & F. Peri; Front. Immunol. 11, 1210 (2020)
- Targeting TLR4-dependent inflammation in post-hemorrhagic brain injury: J.K. Karimy, et al.; Exp. Opin. Ther. Targets 24, 525 (2020) (Review)
- Platelets to surrogate lung inflammation in COVID-19 patients: H.K. Bhotla, et al.; Medic. Hypothes. 143, 110098 (2020)