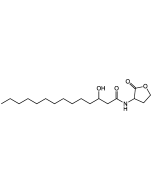Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
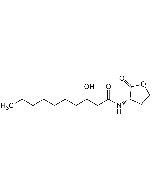
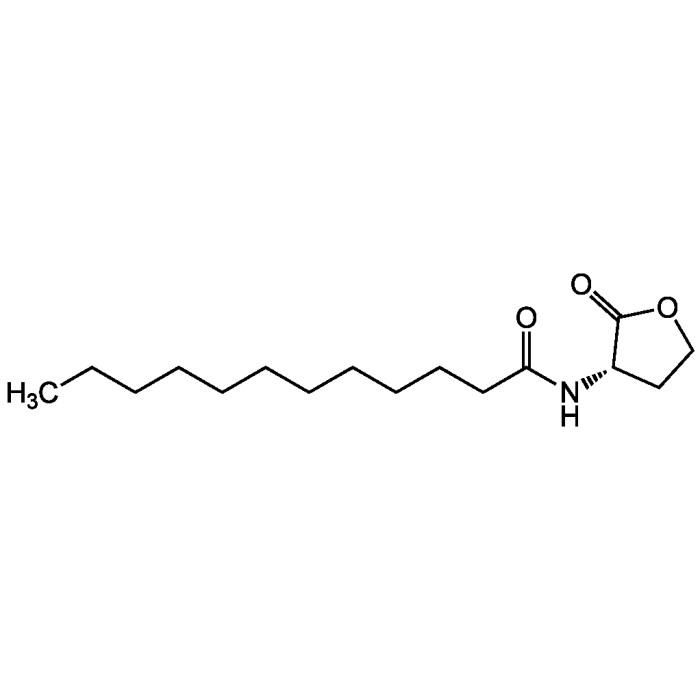
N-Dodecanoyl-L-homoserine lactone
| Product Details | |
|---|---|
| Synonyms | N-[(3S)-Tetrahydro-2-oxo-3-furanyl]-dodecanamide; dDHL; C12-HSL |
| Product Type | Chemical |
| Properties | |
| Formula | C16H29NO3 |
| MW | 283.4 |
| CAS | 137173-46-7 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO and dimethylformamide (approx. 30mg/ml). Ethanol or methanol are not recommended as solvents as they have been shown to open the lactone ring. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WILLZMOKUUPJSL-AWEZNQCLSA-N |
| Smiles | CCCCCCCCCCCC(=O)N[C@H]1CCOC1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
N-Dodecanoyl-L-homoserine lactone (C12-HSL) is a small diffusible signalling molecule involved in quorum sensing, thereby controlling gene expression and affecting cellular metabolism in bacteria. In addition to regulating bacterial functions, C12-HSL activates NF-κB in RAW 264.7 macrophages, increasing the expression of TNF-α, interleukin-1β (IL-1β) and IL-8, while other lactones do not. In addition, C12-HSL alters cell cycling and metabolism of human keratinocyte (HaCaT) cells. It is important to note that C12-HSL is distinct from N-3-oxo-dodecanoyl-L-homoserine lactone, which is produced at different times in biofilm development and has different cellular effects.
(1) G.D. Geske; JACS 127, 12762 (2005) | (2) J.T. Hodgkinson; Tetrahedron Lett. 52, 3291 (2011) | (3) A. Kuo, et al.; J. Bacteriol. 176, 7558 (1994) | (4) J.K. Lithgow, et al.; Mol. Microbiol. 37, 81 (2000) | (5) K.H. McClean, et al.; Microbiol. 143, 3703 (1997) | (6) K. Gomi, et al.; Infect. Immun. 74, 7029 (2006) | (7) S. Kristiansen, et al.; APMIS 116, 361 (2008) | (8) Y. Huang, et al.; ISME 1, (2008)