Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
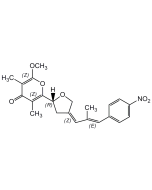
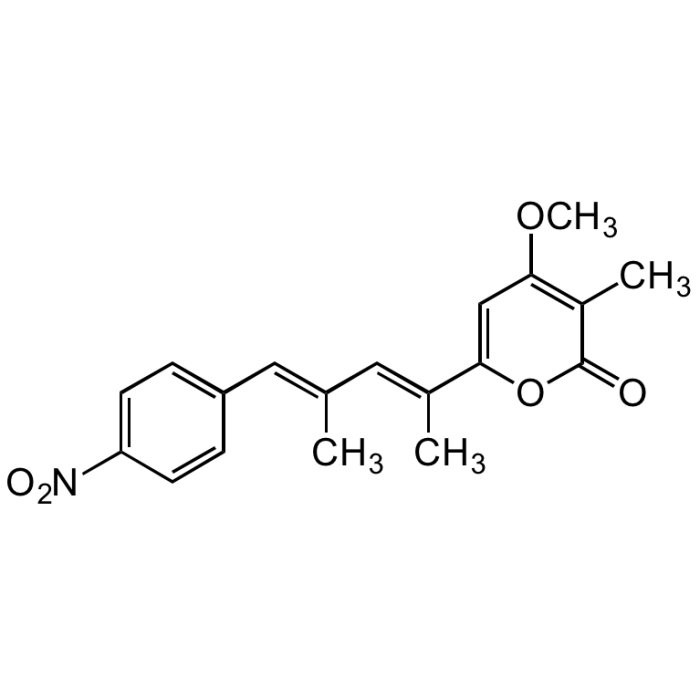
Luteoreticulin
As low as
170
CHF
CHF 170.00
In stock
Only %1 left
BVT-0457-C500500 µgCHF 170.00
BVT-0457-M0011 mgCHF 300.00
| Product Details | |
|---|---|
| Synonyms | Griseulin; 6-[1,3-Dimethyl-4-(4-nitrophenyl)-1,3-butadienyl]-4-methoxy-3-methyl-2H-pyran-2-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C19H19NO5 |
| MW | 341.4 |
| CAS | 22388-89-2 |
| Source/Host Chemicals | Isolated from Streptomyces thioluteus. |
| Purity Chemicals | ≥98% (NMR, HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in DMSO, acetone or chloroform. |
| Identity | Determined by 1H-NMR and MS. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | INCHGEJHIFBBOR-DSEBWEOJSA-N |
| Smiles | COC1=C(C)C(=O)OC(=C1)C(\C)=C\C(\C)=C\C1=CC=C(C=C1)[N+]([O-])=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Nitrophenyl pyranone derivative related to aureothin (Prod. No. BVT-0303).
- Insecticidal and nematocidal compound.
- Metabolites within this product class show pronounced antitumor and immunosuppressive activity.
Product References
- Structure of luteoreticulin, a nitro-containing metabolite of Streptomyces luteoreticuli: Y. Koyama, et al.; Tetrahedron Lett. 1969, 355 (1969)
- Fermentative manufacture of pyrone insecticides: M. G. Nair; PCT Int. Appl. WO 9312656 A1 19930708 (1993)
- Griseulin, a new nitro-containing bioactive metabolite produced by Streptomyces spp: M.G.Nair, et al.; J. Antibiot. (Tokyo) 46, 1762 (1993)
- Structural revision of griseulin, a bioactive pyrone possessing a nitrophenyl unit: Y. Ishibashi, et al.; Chem. Lett. 23, 1747 (1994)
- Convergent syntheses of luteoreticulin and didemethylluteoreticulin: J. W. Lyga; J. Heterocyclic Chem. 32, 515 (1995)
- Rational design of modular polyketide synthases: Morphing the aureothin pathway into a luteoreticulin assembly line: Y. Sugimoto, et al.; Angew. Chem., Int. Edit. 53, 1560 (2014)