Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
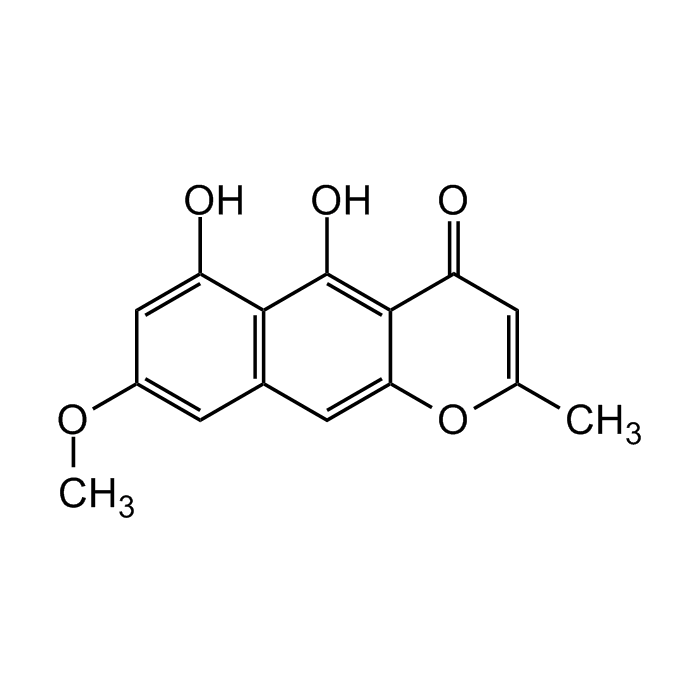
Rubrofusarin
As low as
130
CHF
CHF 130.00
In stock
Only %1 left
BVT-0395-M0011 mgCHF 130.00
BVT-0395-M0055 mgCHF 395.00
| Product Details | |
|---|---|
| Synonyms | NSC 258316 |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H12O5 |
| MW | 272.3 |
| CAS | 3567-00-8 |
| Source/Host Chemicals | Isolated from Fusarium graminearum. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Orange needles. |
| Solubility | Soluble in DMSO. Sparingly soluble in usual organic solvents. |
| Identity | Determined by 1H-NMR and UV. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | FPNKCZKRICBAKG-UHFFFAOYSA-N |
| Smiles | COC1=CC2=C(C(O)=C1)C(O)=C1C(=O)C=C(C)OC1=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Monomer of aurofusarin.
- Mycotoxin.
- Anticancer and antimycobacterial compound in vivo.
- Moderate tyrosinase inhibitor.
- Anti-estrogenic.
Product References
- Structure of rubrofusarin: G.H. Stout, et al.; Chem. Indust. 1961, 289 (1961).
- Metabolic products of fungi. XXV. Synthesis of rubrofusarin and its derivatives: S. Shibata, et al.; Chem. Pharm. Bul.l (Tokyo) 15, 1757 (1967)
- The biosynthesis of rubrofusarin, a polyketide naphthopyrone from Fusarium culmorum: F.J. Leeper & J. Staunton; J. Chem. Soc. Perkin 1 1984, 2919 (1984).
- Biometic syntheses of the polyketide fungal metabolites alternariol and rubrofusarin: C. Abell, et al.; J. Chem. Soc. Chem. Comm. 1986, 15 (1986)
- Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant St. aureus: T. Hatano, et al.; Chem. Pharm. Bull. 47, 1121 (1999)
- A new naphthopyrone derivative from Cassia quinquangulata and structural revision of quinquangulin and its glycosides: X.C. Li, et al.; J. Nat. Prod. 64, 1153 (2001)
- Antimycobacterial naphthopyrones from Senna oblique: J.G. Graham, et al.; J. Nat. Prod. 67, 225 (2004)
- Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents: A.M. El-Halawany, et al.; Chem. Pharm. Bull. (Tokyo) 55, 1476 (2007)
- In vitro cytotoxicity of fungal spoiling maize silage: R.R. Rasmussen, et al.; Food Chem. Toxicol. 49, 31 (2010)
- Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata: H.-B. Huang, et al.; Planta Med 76, 1888 (2010)
- Two novel classes of enzymes are required for the biosynthesis of aurofusarin in Fusarium graminearum: R.J.N. Frandsen, et al.; J. Biol. Chem. 286, 10419 (2011)