Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
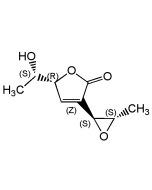
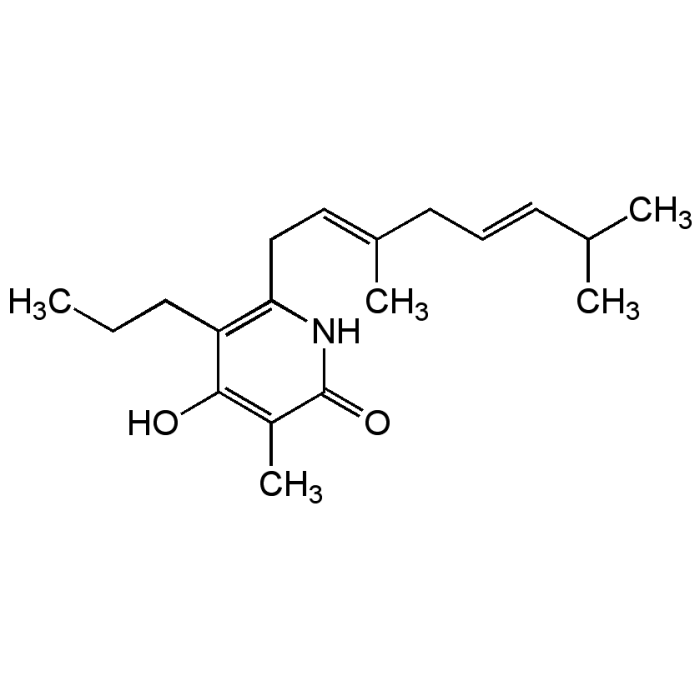
Iromycin A
As low as
215
CHF
CHF 215.00
In stock
Only %1 left
BVT-0262-C500500 µgCHF 215.00
BVT-0262-M0011 mgCHF 310.00
| Product Details | |
|---|---|
| Synonyms | 6-((2E,5E)-3,7-Dimethylocta-2,5-dien-1-yl)-4-hydroxy-3-methyl-5-propylpyridin-2(1H)-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C19H29NO2 |
| MW | 303.4 |
| CAS | 213137-53-2 |
| Source/Host Chemicals | Isolated from Streptomyces bottropensis (Gö-Dra17). |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white to greenish solid. |
| Solubility | Soluble in DMSO; fairly soluble in methanol; poorly soluble in acetone. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | HVAVEUHAOCVIPN-DTCTWCMCSA-N |
| Smiles | CCCC1=C(C\C=C(/C)C\C=C\C(C)C)NC(=O)C(C)=C1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Pyridone metabolite.
- Nitric oxide synthase (NOS) inhibitor, showing selectivity for eNOS (NOS III) versus nNOS (NOS I).
- Thaxtomine biosynthesis inhibitor.
- NADH dehydrogenase (NADH-Coenzyme Q oxidoreductase; Complex I) inhibitor. Inhibits mitochondrial respiration and oxidative phosphorylation (OXPHOS).
Product References
- Iromycins: a new family of pyridone metabolites from Streptomyces sp. II. Convergent total synthesis: H. Shojaei, et al.; J. Org. Chem. 72, 5091 (2007)
- The iromycins, a new family of pyridone metabolites from Streptomyces sp. I. Structure, NOS inhibitory activity, and biosynthesis: F. Surup, et al; J. Org. Chem. 72, 5085 (2007)
- Iromycins from Streptomyces sp. and from synthesis: New inhibitors of the mitochondrial electron transport chain: F. Surup, et al.; Bioorg. Med. Chem. 16, 1738 (2008)