Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
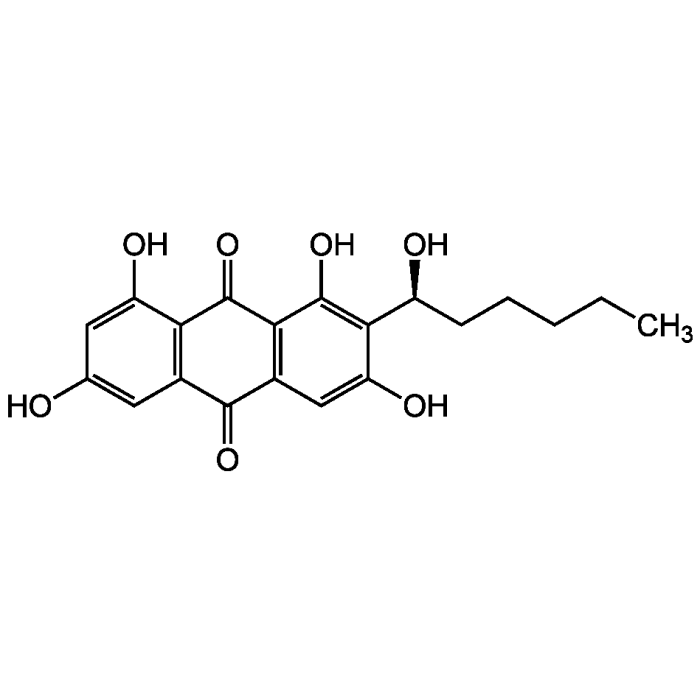
Averantin
As low as
95
CHF
CHF 95.00
In stock
Only %1 left
BVT-0169-M0011 mgCHF 95.00
BVT-0169-M0055 mgCHF 385.00
| Product Details | |
|---|---|
| Synonyms | BRN 2309929; 2-(1-Hydroxyhexyl)-1,3,6,8-tetrahydroxyanthraquinone |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H20O7 |
| MW | 372.4 |
| CAS | 5803-62-3 |
| Source/Host Chemicals | Isolated from Aspergillus sp. (strain WDMH51). |
| Purity Chemicals | ≥98% (HPLC; NMR) |
| Appearance | Orange powder. |
| Solubility | Soluble in acetone, methanol or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | WGPOPPKSQRZUTP-LBPRGKRZSA-N |
| Smiles | CCCCC[C@H](O)C1=C(O)C2=C(C=C1O)C(=O)C1=C(C(O)=CC(O)=C1)C2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Mycotoxin.
- Intermediate of the biosynthetic pathway to aflatoxin B1.
- Cytotoxic against human solid tumor cell lines.
- Moderate antifungal and antibacterial agent.
Product References
- Product B (Averantin), a pigment from Aspergillus versicolor: J.H. Birkinshaw, et al.; J. Chem. Soc. (C) 1966, 855 (1966)
- Stereochemical correlation of (-)-averantin: C.A. Townsend & S.B. Christensen; Tetrahedron Lett. 27, 887 (1986)
- A reappraisal of fungi producing aflatoxins: J. Varga: World Mycotoxin J. 2, 263 (2009)
- Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor: Y.M. Lee, et. al; Arch. Pharm. Res. 33, 231 (2010)
- Genetics of polyketide metabolism in Aspergillus nidulans: M.L. Klejnstrup, et al.; Metabolites 2, 100 (2012)
- The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng: K. Liu, et al.; Nat. Prod. Res. 28, 2334 (2014)