Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
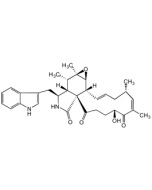
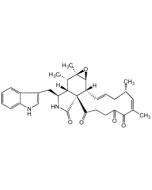
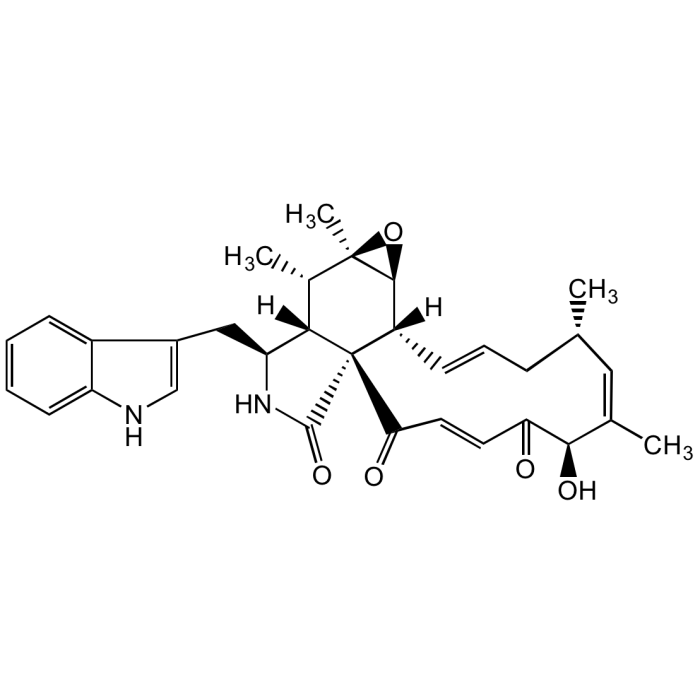
Chaetoglobosin A
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
BVT-0092-M0011 mgCHF 90.00
BVT-0092-M0055 mgCHF 335.00
| Product Details | |
|---|---|
| Synonyms | NSC366739; BRN1097707 |
| Product Type | Chemical |
| Properties | |
| Formula |
C32H36N2O5 |
| MW | 528.7 |
| CAS | 50335-03-0 |
| Source/Host Chemicals | Isolated from Chaetomium sp. |
| Purity Chemicals | ≥80% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in methanol; almost insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | OUMWCYMRLMEZJH-HSXMBTRQSA-N |
| Smiles | [H][C@]12[C@H](CC3=CNC4=CC=CC=C34)NC(=O)[C@@]11C(=O)\C=C\C(=O)[C@H](O)\C(C)=C/[C@@H](C)C\C=C\[C@]1([H])[C@@H]1O[C@]1(C)[C@H]2C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Cytochalasan analog with anti-fungal activity.
- Phytotoxic, nematicidal and anti-bacterial activity.
- Exhibits cytotoxic effects against human cancer cell lines.
- Induces apoptosis in lymphocytic leukemia cells by targeting the cytoskeleton.
- Enhances fibrinolytic activity of bovine aortic endothelial cells.
- Mycotoxin.
Product References
- Acute toxic effects of chaetoglobosin A, a new cytochalasan compound produced by Chaetomium globosum, on mice and rats: K. Ohtsubo, et al.; Jpn. J. Exp. Med. 48, 105 (1978)
- Chaetoglobosins, cytotoxic 10-(indol-3-yl)-[13]cytochalasans from Chaetomium spp. I. Production, isolation and some cytological effects of chaetoglobosins A-J: S. Sekita, et al.; Chem. Pharm. Bull. 30, 1609 (1982)
- Enhancement of fibrinolytic activity of vascular endothelial cells by chaetoglobosin A, crinipellin B, geodin and triticone B: C. Shinohara, et al.; J. Antibiot. 53, 262 (2000)
- Phytotoxic chaetoglobosins are produced by the plant pathogen Calonectriam organii (anamorph Cylindrocladium scoparium): C. Von Wallbrunn, et al.; J. Gen. Appl. Microbiol. 47, 33 (2001)
- Chaetoglobosins Q, R, and T, three further new metabolites from Chaetomium globosum: W. Jiao, et al.; J. Nat. Prod. 67, 1722 (2004)
- Growth and mycotoxin production by Chaetomium globosum: M.R. Fogle, et al.; Mycopathologia 164, 49 (2007)
- New azaphilones from Chaetomium globosum isolated from the built environment: D. R. McMullin, et al.; Tetrahedron Lett. 54, 568 (2013)
- Combinatorial generation of complexity by redox enzymes in the chaetoglobosin A biosynthesis: K. Ishiuchi, et al.; JACS 135, 7371 (2013)
- Nematicidal activity of chaetoglobosin A produced by Chaetomium globosum NK102 against Meloidogyne incognita: Y. Hu, et al.; J. Agricult. Food Chem. 61, 41 (2013)
- Efficacy assessment of antifungal metabolites from Chaetomium globosum No.05, a new biocontrol agent, against Setosphaeria turcica: G. Zhang, et al.; BioControl 64, 90 (2013)
- Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton: P. B. Knudson, et al.; Leukemia 28, 1289 (2014)
- Production of vineomycin A1 and chaetoglobosin A by Streptomyces sp. PAL114: A. Aouiche, et al.; Ann. Microbiol. 65, 1351 (2015)