Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
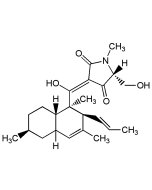
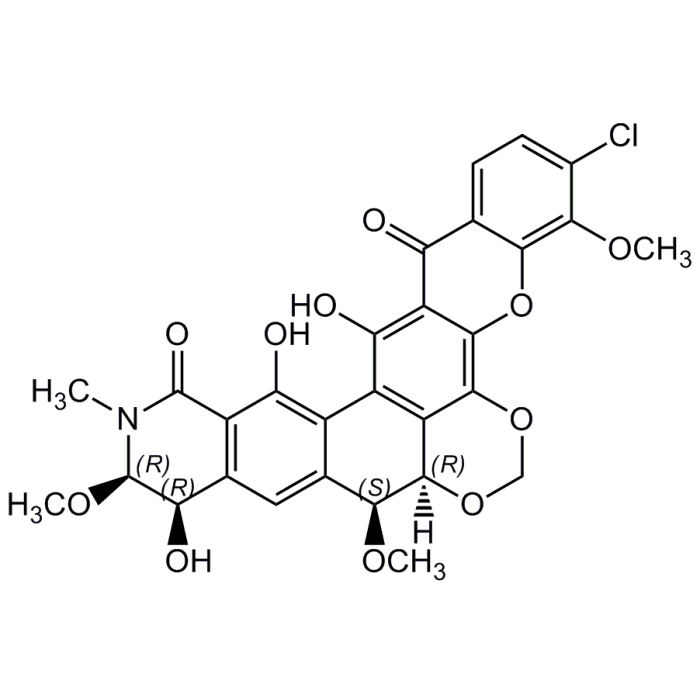
Lysolipin I
As low as
120
CHF
CHF 120.00
In stock
Only %1 left
BVT-0037-C500500 µgCHF 120.00
BVT-0037-M0011 mgCHF 205.00
BVT-0037-M0055 mgCHF 610.00
| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula |
C29H24ClNO11 |
| MW | 597.9 |
| CAS | 59113-57-4 |
| Source/Host Chemicals | Isolated from Streptomyces violaceoniger. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow needles. |
| Solubility | Soluble in methylene chloride, DMSO or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | NEOMIZJYHXSRLV-GLWRQVQYSA-N |
| Smiles | [H][C@]12OCOC3=C4OC5=C(OC)C(Cl)=CC=C5C(=O)C4=C(O)C(C4=C(O)C5=C(C=C4[C@@H]1OC)[C@@H](O)[C@@H](OC)N(C)C5=O)=C23 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Glycopeptide synthesis inhibitor.
- Antibacterial, antifungal and anticoccidial.
- Cytotoxic.
Product References
- Lysolipin I, ein neuer Hemmstoff der bakteriellen Zellwandsynthese.: H. Drautz, et al.; Path. Microbiol. 42, 236 (1975)
- Metabolic products of microorganisms, 149. Lysolipin I, a new antibiotic from streptomyces violaceoniger: H. Drautz, et al.; Arch. Microbiol. 106, 175 (1975)
- Structure of cervinomycin, a novel xantone antibiotic active against anaerobe and mycoplasma.: A. Nakagawa, et al.; J Antibiot 40, 301 (1987)
- Biosynthetic studies on the xanthone antibiotics lysolipins X and I: H. Bockholt, et al.; J. Org. Chem. 59, 2064 (1994)
- Experiments on the total synthesis of Lysolipin I. Part II. Michael addition of 1,3-cyclohexanedione to quinone acetals: R.O. Duthaler & U.H.U. Wegmann; Helv. Chim. Acta 67, 1755 (2004)
- Isolation of the lysolipin gene cluster of streptomyces Tandae Tu 4042: P. Lopez, et al.; Gene 461, 5 (2010)