Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
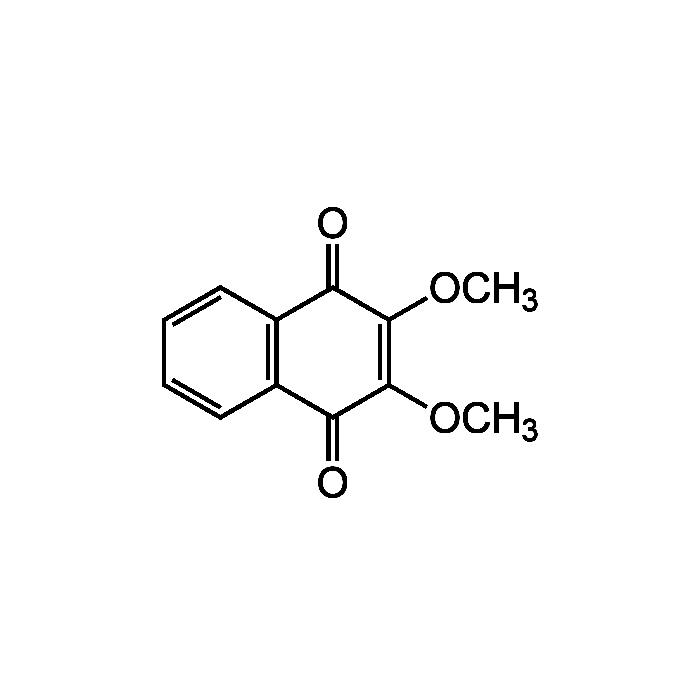
DMNQ
As low as
80
CHF
CHF 80.00
In stock
Only %1 left
AG-CR1-3598-M0055 mgCHF 80.00
AG-CR1-3598-M01010 mgCHF 130.00
AG-CR1-3598-M02525 mgCHF 260.00
| Product Details | |
|---|---|
| Synonyms | 2,3-Dimethoxy-1,4-naphthoquinone; 2,3-diOMe-1,4-NQ |
| Product Type | Chemical |
| Properties | |
| Formula |
C12H10O4 |
| MW | 218.2 |
| CAS | 6956-96-3 |
| Purity Chemicals | ≥99% (NMR) |
| Appearance | Yellow crystalline solid. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| InChi Key | ZEGDFCCYTFPECB-UHFFFAOYSA-N |
| Smiles | COC1=C(OC)C(=O)C2=C(C=CC=C2)C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Cell permeable, non-alkylating, non-thiol, adduct-forming, redox cycling quinone.
- Intracellular superoxide anion formation/ROS generation inducer.
- Anticancer agent. Shown to induce cell proliferation, apoptosis, necrosis and necroptosis in vitro, dependent on concentration, time, temperature and cell type.
- Valuable tool for the generation of reactive oxygen species (ROS) in order to study the role of ROS in cell toxicity, apoptosis and necrosis.
- Useful as reference compound in characterizing the effects of oxidative stress. Can be used to eliminate any mechanistic ambiguity involving redox cycling quinoids as the source of reactive oxidant species/oxidative stress in biological studies.
Product References
- The role of oxidative processes in the cytotoxicity of substituted 1,4-naphthoquinones in isolated hepatocytes: D. Ross, et al.; Arch. Biochem. Biophys. 248, 460 (1986)
- Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes: T.W. Gant, et al.; Chem. Biol. Interact. 65, 157 (1988)
- Quinone-induced DNA single strand breaks in rat hepatocytes and human chronic myelogenous leukaemic K562 cells: W.A. Morgan, et al.; Biochem. Pharmacol. 44, 215 (1992)
- Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells: M.M. Shi, et al.; J. Biol. Chem. 269, 26512 (1994)
- Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells: J.M. Dypbukt, et al.; J. Biol. Chem. 269, 30553 (1994)
- DNA single-strand breakage in mammalian cells induced by redox cycling quinones in the absence of oxidative stress: W.A. Morgan; J. Biochem. Toxicol. 10, 227 (1995)
- Naphthoquinone-induced DNA damage in the absence of oxidative stress: W.A. Morgan; Biochem. Soc. Trans. 23, 225S (1995)
- Differential mechanisms of cell killing by redox cycling and arylating quinones: T.R. Henry & K.B. Wallace; Arch. Toxicol. 70, 482 (1996)
- Naphthazarin derivatives: synthesis, cytotoxic mechanism and evaluation of antitumor activity: Y.J. You, et al.; Arch. Pharm. Res. 21, 595 (1998)
- Temperature-dependent quinone cytotoxicity in platelets involves arylation: Y.A. Kang, et al.; J. Toxicol. Environ. Health A 65, 1367 (2002)
- Superoxide targets calcineurin signaling in vascular endothelium: D. Namgaladze, et al.; BBRC 334, 1061 (2005)
- Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype: M.R. King, et al.; J. Immunol. 176, 2765 (2006)
- Caspase-2 activation in neural stem cells undergoing oxidative stress-induced apoptosis: C. Tamm, et al.; Apoptosis 13, 354 (2008)
- A Receptor-interacting Protein 1 (Rip1) Independent Necrotic Death Under The Control Of Protein Phosphatase Pp2a That Involves The Reorganization Of Actin Cytoskeleton And The Action Of Cofilin-1: A. Tomasella, et al.; J. Biol. Chem. 289, 25699 (2014)
- Airway epithelial Paraoxonase-2 in obese asthma: D.E. Winnica, et al.; PLoS One 17, e0261504 (2022)