Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
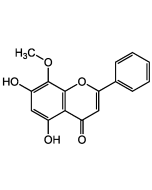
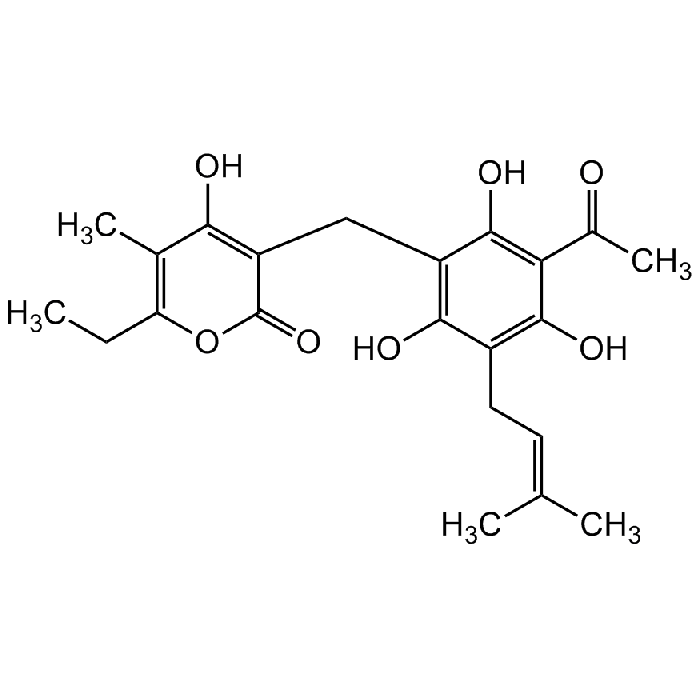
Arzanol
As low as
160
CHF
CHF 160.00
In stock
Only %1 left
AG-CN2-0500-M0011 mgCHF 160.00
| Product Details | |
|---|---|
| Synonyms | Homoarenol; 3-[[3-Acetyl-2,4,6-trihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H26O7 |
| MW | 402.4 |
| CAS | 32274-52-5 |
| Source/Host Chemicals | Isolated from Helichrysum italicum. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | Off-white solid to pale yellow solid. |
| Solubility | Soluble in DMSO, dichloromethane, acetone or 100% ethanol. Poorly soluble in water. |
| Identity | Determined by 1H-NMR. |
| InChi Key | ZOIAPLVBZQQHCG-UHFFFAOYSA-N |
| Smiles | CC1=C(CC)OC(C(CC2=C(O)C(C/C=C(C)/C)=C(O)C(C(C)=O)=C2O)=C1O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent inhibitor of human dihydroorotate dehydrogenase (hDHODH), a key enzyme in de novo pyrimidine biosynthesis. hDHODH is a validated target for the treatment of autoimmune diseases, including multiple sclerosis, lupus, and rheumatoid arthritis, and it has also been associated with the treatment of both solid and liquid cancers. Inhibition of hDHODH is also interesting as a host-targeting antiviral (HTA) strategy, and Arzanol exhibited antiviral activity in SARS-CoV-2-infected cell models.
- Brain glycogen phosphorylase (bGP) agonist. Arzanol directly interacts with bGP, competing for the same allosteric binding site on bGP like AMP (but with higher affinity), inducing similar conformational changes and promoting the transition to the active form, consequently leading to increased bGP enzyme activity.
GPs are key enzymes in glycogen metabolism, promoting the rate-limiting step of its mobilization. In brain, glycogen acts as an emergency glucose storage to protect neurons against hypoglycemia and hypoxic stress, being critical for high cognitive processes such as learning and memory consolidation. Reduced glycogen breakdown is associated with impaired cognitive functions and neuro-degeneration. Activation of glycogen breakdown might be a new therapeutic strategy. - Anti-inflammatory compound. Potent dual inhibitor of pro-inflammatory transcription factors and inflammatory enzymes.
- Inhibitor of NFκB activation.
- Potent inhibitor of inducible microsomal prostaglandin E2 synthase-1 (mPGES-1), the terminal synthase responsible for the synthesis of the pro-tumorigenic prostaglandin E2 (PGE2).
- Antioxidant. 5-Lipoxygenase (5-LO) inhibitor.
- Antibacterial compound.
- HIV-1 replication inhibitor.
Product References
- Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol alpha-Pyrone from Helichrysum italicum ssp. microphyllum: G. Appendino, et al.; J. Nat. Prod. 70, 608 (2007)
- Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated alpha-pyrone-phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum: A. Rosa, et al.; Chem. Biol. Interact. 165, 117 (2007)
- Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo: J. Bauer, et al.; Biochem. Pharmacol. 81, 259 (2011)
- Protective role of arzanol against lipid peroxidation in biological systems: A. Rosa, et al.; Chem. Phys. Lipids 164, 24 (2011)
- A Multicomponent Carba-Betti Strategy to Alkylidene Heterodimers - Total Synthesis and Structure - Activity Relationships of Arzanol: A. Minassi, et al.; Eur. J. Org. Chem. 772 (2012)
- Arzanol, a potent mPGES-1 inhibitor: Novel anti-inflammatory agent: P. S. Kothavade, et al.; Sci. World J. 2013, 986429 (2013) (Review)
- Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum: O. Taglialatela-Scafati, et al.; J. Nat. Prod. 76, 346 (2013)
- Chemoproteomic fishing identifies arzanol as a positive modulator of brain glycogen phosphorylase : F. del Gaudio, et al.; Chem. Commun. 54, 12863 (2018)
- Arzanol Inhibits Human Dihydroorotate Dehydrogenase and Shows Antiviral Activity: M. Alberti, et al.; J. Nat. Prod. (Epub ahead) (2025)