Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
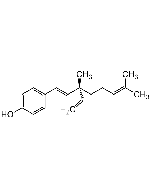
Neobavaisoflavone
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-CN2-0498-M0011 mgCHF 70.00
| Product Details | |
|---|---|
| Synonyms | 7-Hydroxy-3-(4-hydroxy-3-(3-methyl-2-buten-1-yl)phenyl)-4H-1-benzopyran-4-one; 3'-Prenyldaidzein |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H18O4 |
| MW | 322.4 |
| CAS | 41060-15-5 |
| Source/Host Chemicals | Isolated from Psoralea corylifolia sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO, ethanol or methanol. |
| Identity | Determined by 1H-NMR and MS. |
| InChi Key | OBGPEBYHGIUFBN-UHFFFAOYSA-N |
| Smiles | OC1=CC=C(C(C(C2=CC=C(O)C(C/C=C(C)/C)=C2)=CO3)=O)C3=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic. Antibacterial and antifungal compound. Displays antibiotic activity against Gram-negative multidrug resistant bacteria.
- Anticancer compound. DNA polymerase inhibitor. Enhances TRAIL-induced apoptosis via inhibition of metastasis.
- Anti-inflammatory agent inhibiting IL-6-induced STAT3 activation and phosphorylation.
- Platelet aggregation inhibitor.
- Human carboxylesterase 1&2 and UDP-glucuronosyltransferase 1A1 inhibitor, enzymes important in drug metabolism.
- Antioxidant. Inhibits the production of nitric oxide (NO), reactive oxygen species (ROS) and reactive nitrogen species (RNS).
- Neuroprotective. Exerts protective effects against H2O2-induced neuronal cell damage.
- Shows osteogenic acitivty.
Product References
- Prenylated isoflavanone from the roots of Erythrina sigmoidea: A.E. Nkengfack, et al.; Phytochemistry 36, 1047 (1994)
- Antiplatelet flavonoids from seeds of Psoralea corylifolia: W.J. Tsai, et al.; J. Nat. Prod. 59, 671 (1996)
- DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia: N.J. Sun, et al.; J. Nat. Prod. 61, 362 (1998)
- Studies on the chemical constituents of Psoralea corylifolia L.: B. Ruan, et al.; J. Asian Nat. Prod. Res. 9, 41 (2007)
- The combination of TRAIL and isoflavones enhances apoptosis in cancer cells: J. Bronikowska, et al.; Molecules 15, 2000 (2010)
- Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages: E. Szliszka, et al.; Molecules 16, 3701 (2011)
- Neobavaisoflavone stimulates osteogenesis via p38-mediated up-regulation of transcription factors and osteoid genes expression in MC3T3-E1 cells: M.-J. Don, et al.; Phytomed. 19, 551, (2012)
- Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation: S.W. Lee, et al.; Planta Med. 78, 903 (2012)
- Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells: Y.J. Kim, et al.; Life Sci. 95, 101 (2014)
- Activity of three cytotoxic isoflavonoids from Erythrina excelsa and Erythrina senegalensis (neobavaisoflavone, sigmoidin H and isoneorautenol) toward multi-factorial drug resistant cancer cells: V. Kuete, et al.; Phytomed. 21, 682 (2014)
- Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes: A.T. Mbaveng, et al.; SpringerPus 4, 823 (2015)
- Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2: Y.G. Li, et al.; Fitoterapia 101, 99 (2015)
- Identification and characterization of naturally occurring inhibitors against UDP-glucuronosyltransferase 1A1 in Fructus Psoraleae (Bu-gu-zhi): X.X. Wang, et al.; Toxicol. Appl. Pharmacol. 289, 70 (2015)
- Quantitative analysis of Psoralea corylifolia L. and its neuroprotective and anti-neuroinflammatory effects in HT22 hippocampal cells and BV-2 microglia: Y.J. Kim, et al.; Molecules 21, e2076 (2016)
- Inhibition behavior of fructus psoraleae’s ingredients towards human carboxylesterase 1 (hCES1): D.X. Sun, et al.; Xenobiotica 46, 503 (2016)