Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
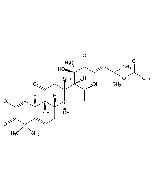
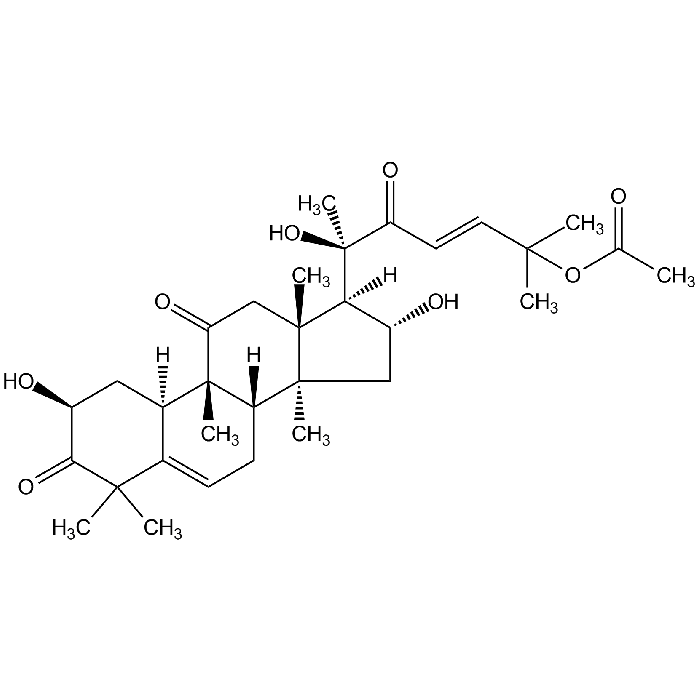
Cucurbitacin B
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CN2-0472-M0011 mgCHF 40.00
AG-CN2-0472-M0055 mgCHF 110.00
AG-CN2-0472-M02525 mgCHF 440.00
| Product Details | |
|---|---|
| Synonyms | Cuc B; 1,2-Dihydro-α-elaterin; Amarine; NSC 49451; NSC 144154 |
| Product Type | Chemical |
| Properties | |
| Formula |
C32H46O8 |
| MW | 558.7 |
| CAS | 6199-67-3 |
| Source/Host Chemicals | Isolated from Cucumis melo L. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white powder. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| InChi Key | IXQKXEUSCPEQRD-DKRGWESNSA-N |
| Smiles | [H][C@@]1([C@H](O)C[C@@]2(C)[C@]3([H])CC=C4[C@@]([H])(C[C@H](O)C(=O)C4(C)C)[C@]3(C)C(=O)C[C@]12C)[C@@](C)(O)C(=O)\C=C\C(C)(C)OC(C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Microtubule polymerization inhibitor. Disrupts F-actin and induces nucleophosmin/B23 translocation.
- Immunomodulator with antimicrobial, anti-inflammatory and antitumorigenic properties.
- Induces apoptosis, autophagy and cell cycle arrest at G2/M phase in a range of cancer cell lines.
- Shown to inhibit STAT 3 phosphorylation and expression levels and blocks JAK2 activity, as well as the transcriptional activity of HIF1α and NF-κB.
- Antioxidant.
- Serves as an ecdysteroid receptor antagonist in Drosophila.
Product References
- Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor: L. Dinan, et al.; Biochem. J. 327, 643 (1997)
- Cucurbitacin glucosides: antioxidant and free-radical scavenging activities: T. Tannin-Spitz, et al.; BBRC 364, 181 (2007)
- Cucurbitacin B induces differentiation, cell cycle arrest, and actin cytoskeletal alterations in myeloid leukemia cells: T. Haritunians, et al.; Leuk. Res. 32, 1366 (2008)
- Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme: D. Yin, et al.; Int. J. Cancer 123, 1364 (2008)
- Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo: N. Wakimoto, et al.; Cancer Sci. 99, 1793 (2008)
- Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562: K.T. Chan, et al.; Cancer Lett. 289, 46 (2010)
- Cucurbitacin B induces rapid depletion of the G-actin pool through reactive oxygen species-dependent actin aggregation in melanoma cells: Y. Zhang, et al.; Acta Biochim. Biophys. Sin. (Shanghai) 43, 556 (2011)
- Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus: S. Dakeng, et al.; J. Cell Biochem. 113, 49 (2012)
- Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis: T. Zhang, et al.; Autophagy 8, 559 (2012)
- Cucurbitacin B inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/B23 translocation: S. Duangmano, et al.; BMC Complement. Altern. Med. 12, 185 (2012)
- Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1α: J. Ma, et al.; Eur. J. Pharmacol. 723, 46 (2014)
- Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells: J. Guo, et al.; Anticancer Agents Med. Chem. 14, 1146 (2014)
- Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells: M. Zhang, et al.; Mol. Med. Rep. 10, 2905 (2014)
- Inhibitory effect of cucurbitacin B on imiquimod-induced skin inflammation: Z.J. Li, et al.; BBRC 459, 673 (2015)
- Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages: M. Kim, et al.; Immunopharmacol. Immunotoxicol. 37, 473 (2015)