Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
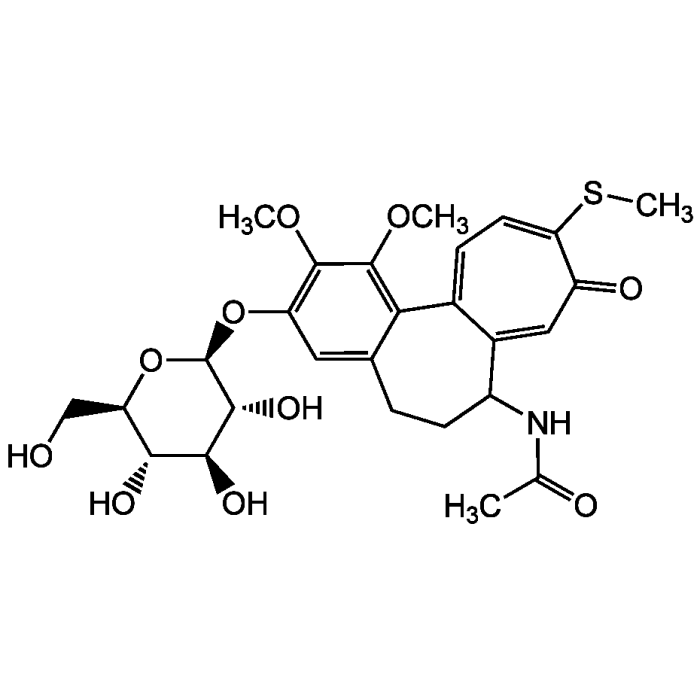
Thiocolchicoside
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CN2-0076-M0011 mgCHF 40.00
AG-CN2-0076-M0055 mgCHF 70.00
AG-CN2-0076-M02525 mgCHF 170.00
| Product Details | |
|---|---|
| Synonyms | BRN 0072205; NSC 147755; Coltramyl; 10-thio-Colchicoside; 2-Demethoxy-2-glucosidoxythiocolchicine |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H33NO10S |
| MW | 563.6 |
| Merck Index | 14: 9324 |
| CAS | 602-41-5 |
| Source/Host Chemicals | Semisynthetic. |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Yellow solid. |
| Solubility | Soluble in water or ethanol. |
| Identity | Determined by 1H-NMR. |
| InChi Key | LEQAKWQJCITZNK-MSQQGMGVSA-N |
| Smiles | COC1=C(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)C=C2CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C3C2=C1OC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent competitive γ-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist.
- Weak nicotinic acetylcholine receptor agonist.
- Muscle relaxant.
- Anti-inflammatory. Has analgesic properties.
- Shows strong epileptogenic and convulsant activity.
- Anticancer compound through inhibition of NF-κB and NF-κB-regulated gene products
- Apoptosis inducer.
- Suppressed osteoclastogenesis induced by RANKL and tumor cells via the NF-κB signaling pathway.
- Therapeutic option for the management of bone metastatic disease.
Product References
- Affinity of thiocolchicoside and thiocolchicoside analogues for the postsynaptic GABA receptor site: K. Biziere, et al.; Eur. J. Pharmacol. 75, 167 (1981)
- Review of the toxicology, pharmacodynamics and pharmacokineticss of thiocolchicoside, a GABA-agonist muscle relaxant with anti-inflammatory and analgesic actions: J.M. Janbroers; Acta Ther. 13, 221 (1987)
- Interaction of thiocolchicoside with [3H]strychnine binding sites in rat spinal cord and brainstem: M. Cimino, et al.; Eur. J. Pharmacol. 318, 201 (1996)
- Focal and secondarily generalised convulsive status epilepticus induced by thiocolchicoside in the rat: G. Sechi, et al.; Seizure 12, 508 (2003)
- Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain: F. Tüzün, et al.; Joint Bone Spine 70, 356 (2003)
- The muscle relaxant thiocolchicoside is an antagonist of GABAA receptor function in the central nervous system: M. Carta, et al.; Neuropharmacology 51, 805 (2006)
- Thiocolchicoside inhibits the activity of various subtypes of recombinant GABA(A) receptors expressed in Xenopus laevis oocytes: M.P. Mascia, et al.; Eur. J. Pharmacol. 558, 37 (2007)
- Thiocolchicoside exhibits anticancer effects through downregulation of NF-κB pathway and its regulated gene products linked to inflammation and cancer: S. Reuter, et al.; Cancer Prev. Res. 3, 1462 (2010)
- Thiocolchicoside suppresses osteoclastogenesis induced by RANKL and cancer cells through inhibition of inflammatory pathways: a new use for an old drug: S. Reuter, et al.; Br. J. Pharmacol. 165, 2127 (2012)
- Thiocolchicoside a semi-synthetic derivative of the Glory Lily: a new weapon to fight metastatic bone resorption? O. Micheau, et al.; Br. J. Pharmacol. 165, 2124 (2012)