Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
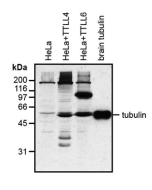
anti-α-Tubulin (acetylated), mAb (TEU318)

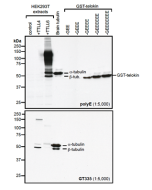
Method: HEK-293T cells grown in standard culture conditions, transfected with plasmids expressing the tubulin acetyl transferase α-TAT1 (or MEC17; J.S. Akella, et al.; Nature 2010; T. Shida, et al.; PNAS 2010) and are lysed in Laemmli sample buffer. Brain tubulin was prepared following standard procedures (M. Castoldi & A.V. Popov; Protein Expr. Purif. 2003). Samples are run on a 10% SDS-PAGE and proteins are transferred to a nitrocellulose membrane and detected by standard immuno blot protocol using anti-α-Tubulin (acetylated), mAb (TEU318) (1:2’000) in TBS containing 0.1% Tween-20 for washing steps and 2.5% fat free milk for antibody incubation. In normal HEK cells, acetylated α-tubulin is detected, although faintly. Expression of α-TAT1 (or MEC17) acetyltransferase leads to enhanced acetylation of tubulin. Brain tubulin is highly acetylated and therefore is strongly detected.
Picture courtesy of Dr. Sudarshan Gadadhar & Dr. Carsten Janke, Curie Institute, Paris
| Product Details | |
|---|---|
| Product Type | Monoclonal Antibody |
| Properties | |
| Clone | TEU318 |
| Isotype | Mouse IgG1 |
| Source/Host | Purified from concentrated hybridoma tissue culture supernatant. |
| Immunogen/Antigen | Tubulin of the Ciliate Euplotes eluted from the 55kDa band of SDS gel. |
| Application |
Western Blot: 1:2000 |
| Crossreactivity | All |
| Specificity |
Detects K40 acetylation of α-tubulin; signal specifically increased by modification with tubulin acetyl transferase α-TAT1. |
| Purity | ≥95% (SDS-PAGE) |
| Purity Detail | Protein G-affinity purified. |
| Concentration | 1mg/ml |
| Formulation | Liquid. In PBS containing 10% glycerol and 0.02% sodium azide. |
| Isotype Negative Control | |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After opening, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Microtubules are key elements of the eukaryotic cytoskeleton that dynamically assemble from heterodimers of α- and β-tubulin. Two different mechanisms can generate microtubule diversity: the expression of different α- and β-tubulin genes, referred to as tubulin isotypes, and the generation of posttranslational modifications (PTMs) on α- and β-tubulin. Tubulin PTMs include the well-known acetylation or phosphorylation, and others that have so far mostly been found on tubulin, detyrosination/tyrosination, polyglutamylation and polyglycylation. These PTMs might have evolved to specifically regulate tubulin and microtubule functions. Tubulin acetylation was discovered on K40 of flagellar α-tubulin in Chlamydomonas reinhardtii and is generally enriched on stable microtubules in cells. It is located on the microtubule lumenal surface. As a result of its localization at the inner face of microtubules, K40 acetylation might rather affect the binding of microtubule inner proteins, a poorly characterized family of proteins. Functional experiments in cells have further suggested that K40 acetylation regulates intracellular transport by regulating the traffic of kinesin motors probably by indirect mechanisms. Acetyltransferase α-Tat1 (or Mec-17) specifically acetylate α-tubulin K40. Acetylation of tubulin by α-Tat1 accumulates selectively in stable, long-lived microtubules thus explaining the link between this posttranslational modication and stable microtubules in cells. However, the direct cellular function of K40 acetylation on microtubules is still unclear.
- Isolation and characterization of libraries of monoclonal antibodies directed against various forms of tubulin in Paramecium: A.M. Callen, et al.; Biol. Cell 81, 95 (1994)
- Where and when is microtubule diversity generated in Paramecium? Immunological properties of microtubular networks in the interphase and dividing cells: A. Fleury, et al.; Protoplasma 189, 37 (1995)
- Structural inheritance in Paramecium: ultrastructural evidence for basal body and associated rootlets polarity transmission through binary fission: F. Iftode & A. Fleury-Aubusson; Biol. Cell 95, 39 (2003)
- Investigating tubulin posttranslational modifications with specific antibodies: M.M. Magiera & C. Janke; In Methods Cell Biol. (Burlington: Academic Press) 115, 247 (2013)