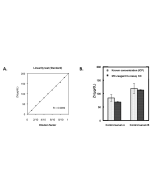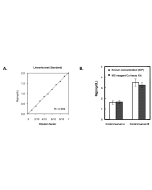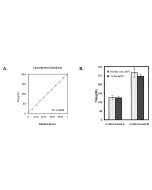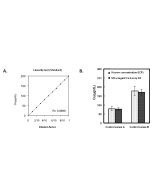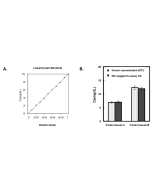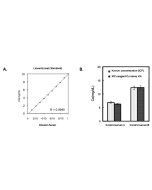Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
JaICA
Iron Colorimetric Assay Kit (Ferrozine Method)

| Product Details | |
|---|---|
| Product Type | Kit |
| Properties | |
| Application Set | Quantitative ELISA |
| Specificity | Specific to Fe2+ and Fe3+. |
| Crossreactivity | All |
| Quantity | Enough reagents for 100 tests. |
| Range | 5 - 1'000μg/dL |
| Sample Type |
Cell Lysate Plasma Saliva Serum Tissue Supernatant Urine |
| Detection Type | Colorimetric |
| Kit Contains |
1 x 20 ml R-1 Buffer (ready to use) 1 x 0.8 ml R-2 Chelate color (ready to use) 1 x 4 ml STD Iron standard 200 µg/dL (ready to use) |
| Other Product Data |
This MC-Reagent Iron Assay Kit is a direct colorimetric assay based on the FerroZine® method without deproteinization of the sample. A weak acid buffer dissociates iron from the transferrin-iron complex and is reduced by a reductant (Ferric >> Ferrous). Ferrous ions form a complex with the chromogen Ferrozine. The color intensity is proportional to the iron concentration in the sample. Absorbance of the Fe2+-complex is measured at 560nm. Wavelength range of sensitivity: 540 ~ 580nm. Features of this Assay: Quick & Easy to use • Species independent • Highly sensitive, stable and suitable for high-throughput testing • No toxic substances • For cell lysates, serum, plasma and wide variety of biological samples |
| Declaration | Manufactured by JaICA. |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Do not freeze. |
| Use/Stability | 12 months after the day of manufacturing. See expiry date on ELISA Kit box. |
| Documents | |
| Manual |
 Download PDF Download PDF |
| MSDS | Inquire |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Iron is a mineral (functioning as an enzyme cofactor) that plays an essential role in many biological processes. It is essential to nearly all known organisms. As a transition element it can form a range of oxidation states, the most common being Fe2+ (or ferrous iron) and Fe3+ (or ferric iron). Ingested iron is mainly absorbed in the form of Fe2+. The trivalent form and the heme-bound Fe2+-component of iron is reduced by vitamin C. Before passing into the plasma, it is oxidized by ceruloplasmin to Fe3+ and bound to transferrin to form a transferrin-iron complex. Iron is generally stored in the centre of metalloproteins, in the heme complex, and in oxygen carrier proteins. Iron-containing proteins participate in many reactions, often utilizing transitory changes in the oxidation state of iron to carry out chemical reactions. Iron is important for redox reactions, oxygen transport (e.g. hemoglobin), short-term oxygen storage (e.g. myoglobin) and energy generation. Iron deficiency has many adverse consequences, including anemia, hemochromatosis, chronic renal disease and in children, behavioral and learning disorders. Iron excess is toxic to the body, harming the heart, liver, skin, pancreatic islet beta cells, bones, joints, and pituitary gland. Maintaining proper iron balance is essential for maintaining homeostasis and health.
- FecA1, a bacterial iron transporter, determines the survival of Helicobacter pylori in the stomach: H. Tsugawa, et al.; Free Radic. Biol. Med. 52, 1003 (2012)
- Estrogen Regulates Hepcidin Expression via GPR30-BMP6-Dependent Signaling in Hepatocytes: Y. Ikeda, et al.; PLoS One 7, e40465 (2012)
- GRK6 deficiency in mice causes autoimmune disease due to impaired apoptotic cell clearance; M. Nakaya; Nat. Comm. 4, 1532 (2013)
- Dietary iron restriction inhibits progression of diabetic nephropathy in db/db mice: Y. Ikeda; Am. J. Physiol. Renal. Physiol. 304, F1028 (2013)
- Iron Chelation by Deferoxamine Prevents Renal Interstitial Fibrosis in Mice with Unilateral Ureteral Obstruction: Y. Ikeda; PLoS One 9, e89355 (2014)