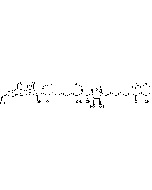Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
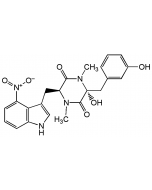
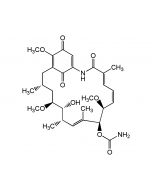
Nargenicin A1
155
CHF
CHF 155.00
In stock
BVT-0204-M0011 mgCHF 155.00
BVT-0204-M0055 mgCHF 505.00

| Product Details | |
|---|---|
| Synonyms | CP-47,444; CS682 |
| Product Type | Chemical |
| Properties | |
| Formula |
C28H37NO8 |
| MW | 515.6 |
| CAS | 70695-02-2 |
| Source/Host Chemicals | Isolated from Actinomyces sp. Gö301. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white powder. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | YEUSSARNQQYBKH-SIMZXIQRSA-N |
| Smiles | [H][C@]12C=C[C@]3([H])C[C@H](OC)C(=O)O[C@]([H])([C@@H](C)O)[C@H](C)\C=C(C)\[C@]33O[C@H]1[C@H](OC(=O)C1=CC=CN1)[C@H](C)[C@@H](O)[C@]23[H] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic against Gram-positive bacteria, particularly Staphylococcus and Clostridia.
- More effective against multi-resistant strains (MRSA) than erythromycin and vancomycin.
- Inhibits cell proliferation and induces HL-60 cell differentiation in combination with 1,25-Dihydroxyvitamin-D3 and ATRA.
- Antioxidant.
Product References
- Structure of natural antibiotic CP-47,444: W.D. Celmer et al., J. Am. Chem. Soc. 102, 4203 (1980)
- Nargenicin biosynthesis: D.E. Cane et al., J. Am. Chem. Soc. 115, 527 (1993)
- The chemistry of the Nargenicin macrolides: J. Kallmerten, in Studies in natural products chemistry (Ed.: A.-U. Rahman, Elsevier) 17, 283 (1995)
- Production, isolation and biological activity of nargenicin from Nocardia sp. CS682: J.K. Sohng et al., Arch. Pharm. Res. 31, 1339 (2008)
- Quantitative analysis of nargenicin in Nocardia sp. CS682 culture by HPLC: S.S. Cho et al., Arch. Pharm. Res. 32, 335 (2009)
- Nargenicin attenuates lipopolysaccharide-induced responses in BV-2 cells: J.C. Yoo et al., Neuroreport 20, 1007 (2009)
- Nargenicin enhances 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced leukemia cell differentiation via PKCbetal/MAPK pathways: S.H. Kim et al., Biochemical Pharmacology 77, 1694 (2009)
- Enhanced production of nargenicin A1 and generation of novel glycosylated derivatives: D. Dhakal, et al.; Appl. Biochem. Biotechnol. 175, 2934 (2015)
- Biosynthesis and ether-bridge formation in nargenicin macrolides: S.J. Pidot, et al.; Angew. Chem. Int. Ed. 58, 3996 (2019)
- Protective effects of nargenicin A1 against tacrolimus-Induced oxidative stress in hirame natural embryo cells: C. Park, et al.; Int. J. Environ. Res. Public Health 16, 1044 (2019)